Quality control method of ginkgolide medicinal preparation
A quality control method and ginkgolide technology are applied in the field of quality control of ginkgolide pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
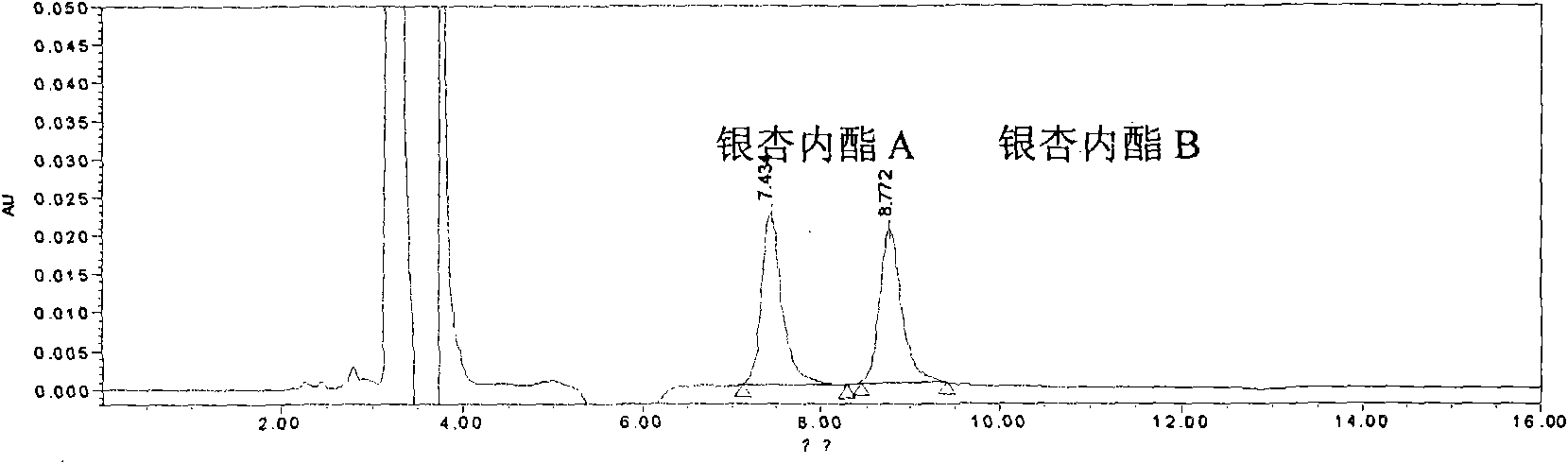
[0175]Embodiment 1: a kind of quality control method of ginkgolide injection, the method comprises the steps:
[0176] High performance liquid chromatography content determination:
[0177] Determination according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2005 edition).
[0178] Chromatographic conditions and system suitability test: filler: silica gel bonded with octadecylsilane; mobile phase: isopropanol: methanol: water = 6:20:70; detection wavelength: 220nm; number of theoretical plates: according to ginkgo biloba Lactone B meter should not be less than 6000.
[0179] Preparation of reference substance solution: Take appropriate amount of reference substances of ginkgolide A, ginkgolide B, and ginkgolide K, weigh them accurately, add 50% acetone and dissolve them to make solutions containing 0.5mg, 0.5mg, and 0.1mg per 1 mL , that is.
[0180] Preparation of the test solution: Accurately measure 2ml of this product and put it in a ...
Embodiment 2
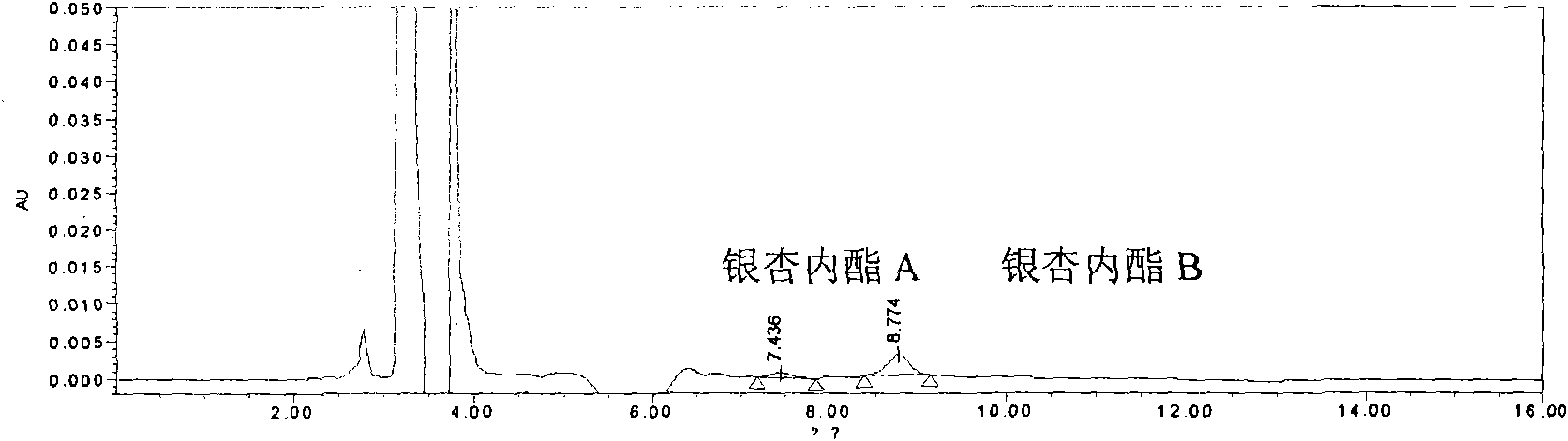
[0183] Embodiment 2: a kind of quality control method of ginkgolide injection, the method comprises the steps:
[0184] High performance liquid chromatography content determination:
[0185] Determination according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2005 edition).
[0186] Chromatographic conditions and system suitability test: filler: octadecylsilane bonded silica gel; mobile phase: isopropanol: methanol: water = 7:21:72; detection wavelength: 220nm; number of theoretical plates: according to Ginkgo Ester B meter should not be less than 7000.
[0187] Preparation of reference substance solution: Take appropriate amount of reference substances of ginkgolide A, ginkgolide B, and ginkgolide K, weigh them accurately, add 50% acetone and dissolve them to make solutions containing 0.5mg, 0.5mg, and 0.1mg per 1 mL , that is.
[0188] Preparation of the test solution: Accurately measure 2ml of this product and place it in a 10ml measur...
Embodiment 3
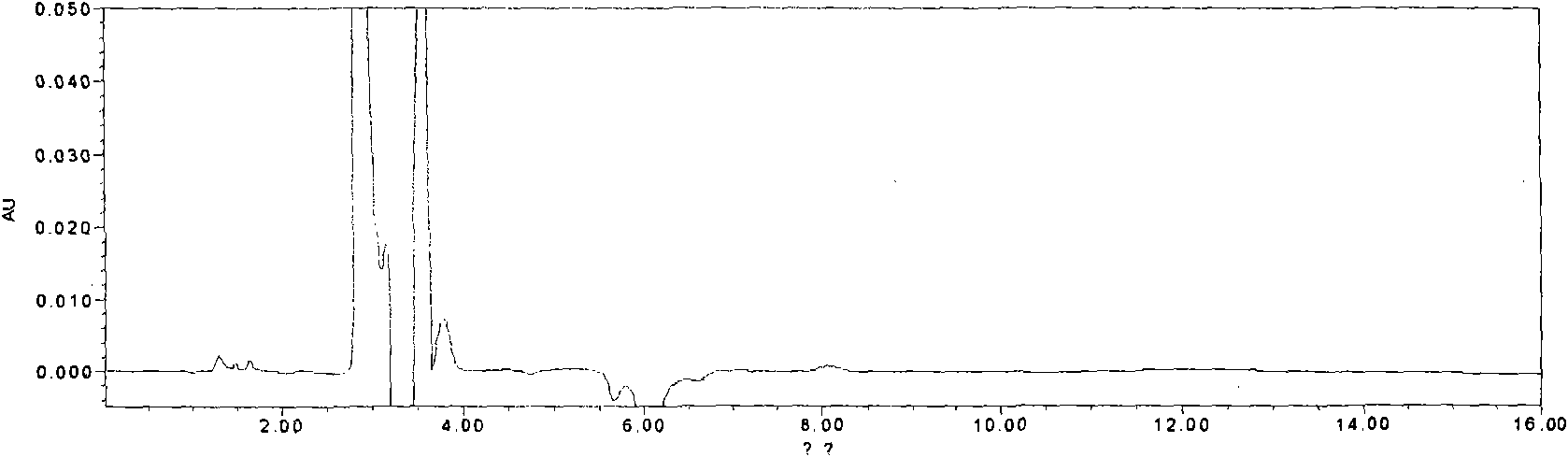
[0191] Embodiment 3: a kind of quality control method of ginkgolide injection, the method comprises the steps:
[0192] High performance liquid chromatography content determination:
[0193] Determination according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2005 edition).
[0194] Chromatographic conditions and system suitability test: filler: octadecylsilane bonded silica gel; mobile phase: isopropanol: methanol: water = 9:25:75; detection wavelength: 220nm; number of theoretical plates: according to Ginkgo biloba Ester B meter should not be less than 7000.
[0195] Preparation of reference substance solution: Take appropriate amount of reference substances of ginkgolide A, ginkgolide B, and ginkgolide K, weigh them accurately, add 50% acetone and dissolve them to make solutions containing 0.5mg, 0.5mg, and 0.1mg per 1 mL , that is.
[0196] Preparation of the test solution: Accurately measure 2ml of this product and place it in a 10ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com