Fixed drug ratios for treatment of hematopoietic cancers and proliferative disorders
A proliferative disease, cancer technology, applied in the fields of application, animal repellent, plant growth regulator, etc., can solve the problems of increased cost, increased risk of infusion complications, and prolonged hospital stay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
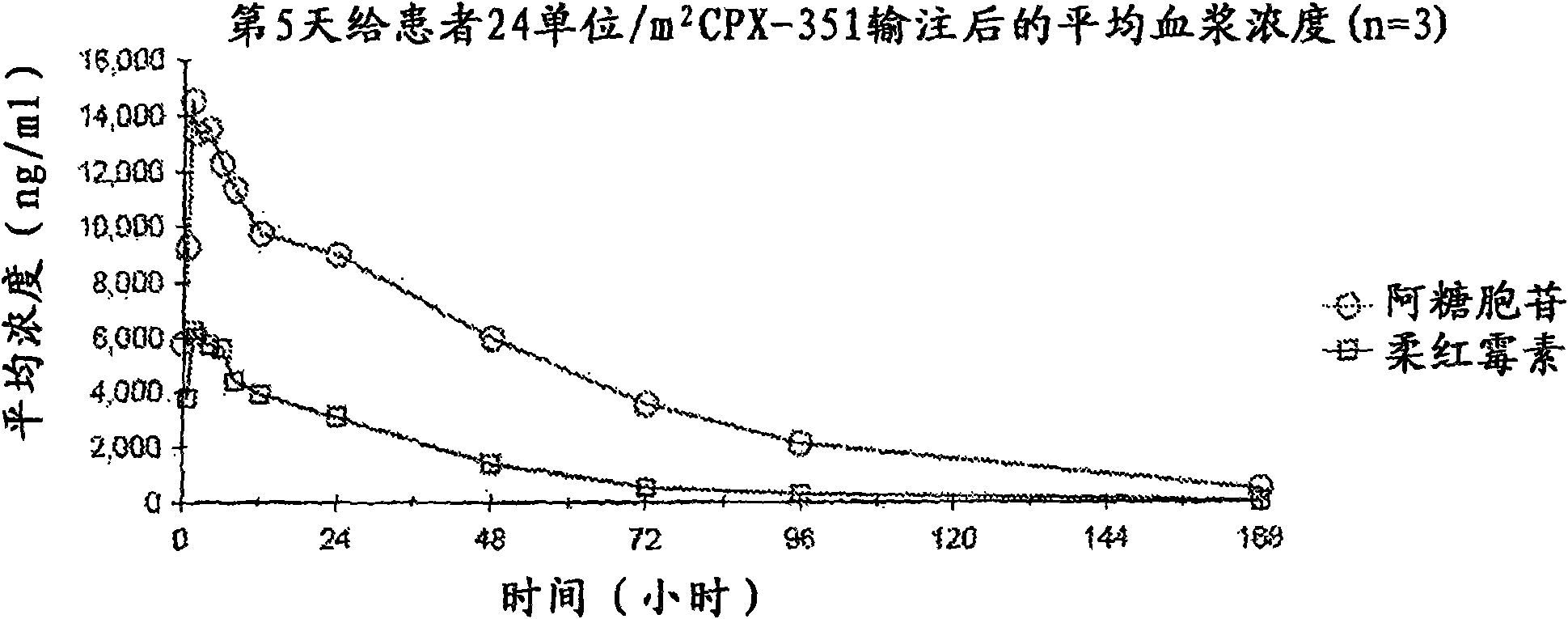
[0044] In Vivo Trials Using CPX-351
[0045] CPX-351 (cytarabine:daunorubicin) liposomal injection has been studied in rats and dogs by single and repeated dose administration (3 doses given every other day, repeated after 2 weeks) of toxicology.
[0046] Single dose study. In the single dose study (Table 1), CPX-351 was administered by intravenous infusion over one hour and the animals were observed for 14 days. "Vehicle control" included liposomes containing copper gluconate but no drug. No drug-related deaths occurred in rats receiving a single dose of CPX-351. Dose-related changes in various hematological parameters and extramedullary hematopoiesis in the spleen and liver were observed in both males and females in the medium and high dose groups compared to control rats. The no observed adverse effect level (NOAEL) single dose of CPX-351 in rats was 10 mg / kg cytarabine: 4.4 mg / kg daunorubicin. In dogs given a single dose of CPX-351, high-dose (6 mg / kg cytarabine: 2...
Embodiment 2
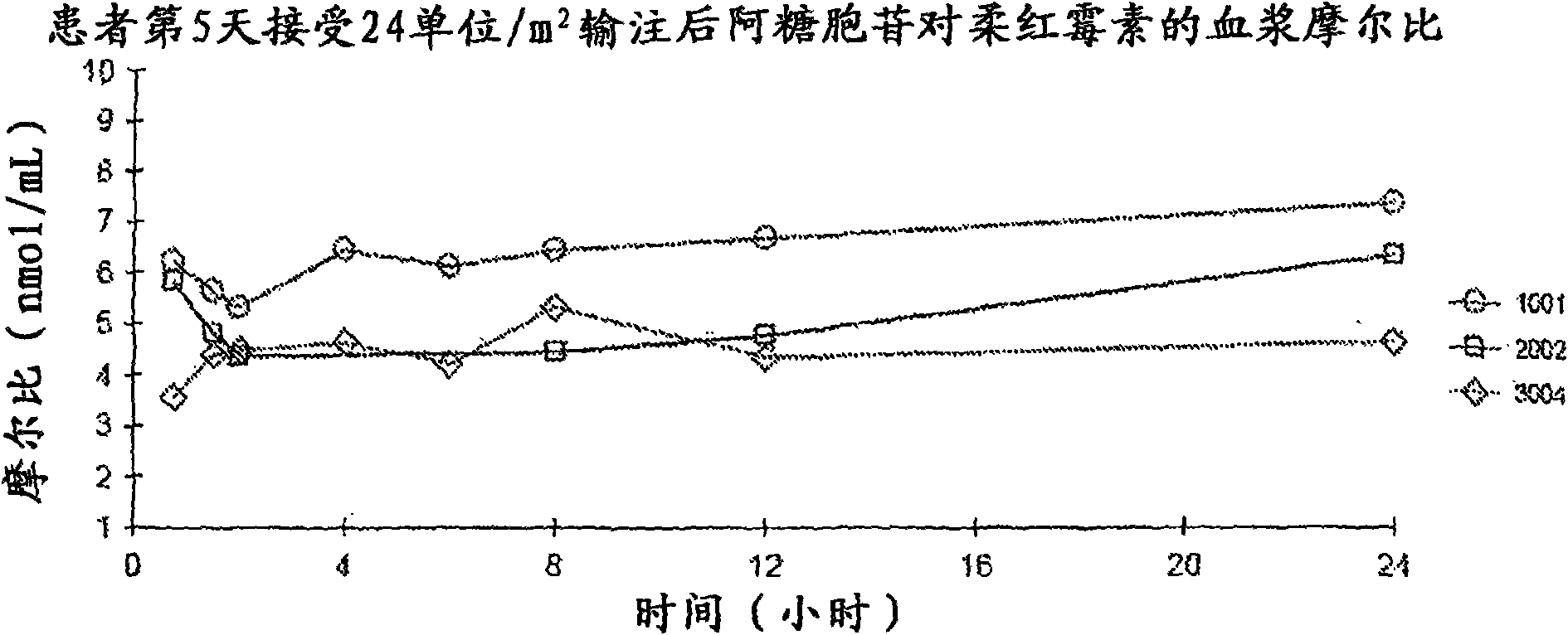
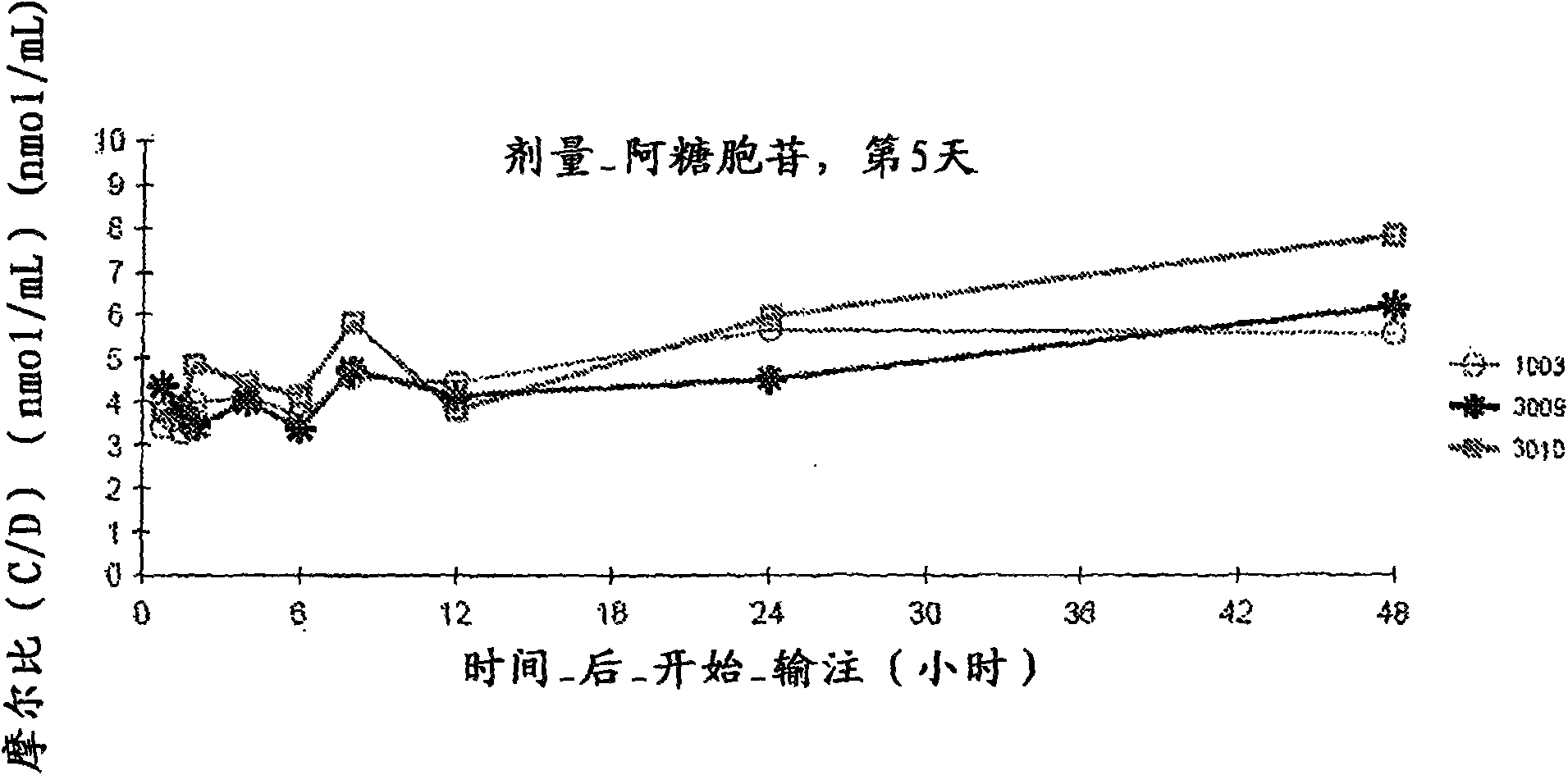
[0055] Phase I clinical trial
[0056] Physical, chemical and drug information. CPX-351 is a liposomal formulation of a fixed combination of the antineoplastic drugs cytarabine and daunorubicin. The two drugs were present in liposomes in a 5:1 molar ratio that showed non-antagonistic activity in preclinical studies. The liposome membrane is composed of distearoylphosphatidylcholine, distearoylphosphatidylglycerol and cholesterol in a molar ratio of 7:2:1. These liposomes have a nominal diameter of approximately 100 nm and are suspended in sucrose-phosphate buffer at pH 7.4. Sterilization was achieved by filtration through a 0.22 μm filter.
[0057] CPX-351 is supplied as a sterile, pyrogen-free, purple opaque 5 mL suspension in single-use 10 mL vials and is also available as a 20 or 25 mL suspension in 50 mL vials. CPX-351 was stored frozen (-20°C) and thawed at room temperature 60 minutes prior to dilution and administration. CPX-351 can also be stored lyophilized and...
Embodiment 3
[0127] Phase I Clinical Trial of CPX-351 - Case Study
[0128] Below are 5 case studies of patients treated in the ongoing Phase I clinical trial of CPX-351.
[0129] case study 1 - Response to CPX-351 in AML patients (even if not responding to prior conventional 7 / 3 cytarabine / daunorubicin therapy): Patient 02-011 is a 62-year-old male diagnosed with AML in 2007 from Received initial Revlimid treatment from September to November 2007 and showed no non-response. In December 2007, the patient was given standard conventional 7+3 cytarabine / daunorubicin therapy and remained unresponsive. Analysis of his bone marrow 14 days after initiation of conventional 7+3 cytarabine / daunorubicin treatment revealed 5% cellularity and 24% blasts. Patients were directly enrolled in the CPX-351 Phase I clinical trial and received 134 units / m on a schedule of days 1, 3, and 5 starting January 2, 2008 2 CPX-351. Analysis of bone marrow after 15 days showed hypoplasia (<10% cellularity, <5% b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com