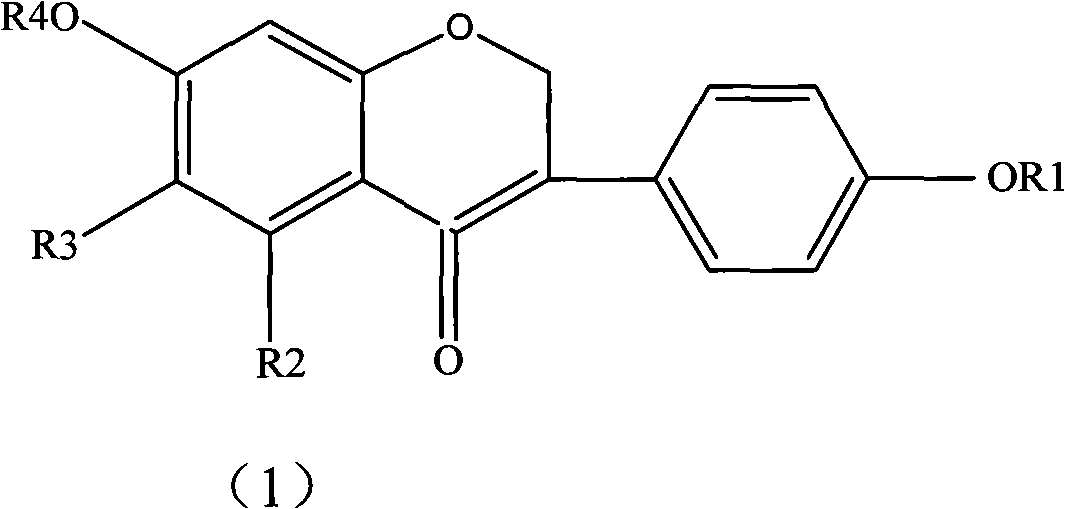
Drug combination containing prunetin and application thereof in drugs
A composition, the technology of prunus, which is applied in the direction of drug combination, medical preparation containing active ingredients, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Estrogen-like activities of pratensein and prunetin
[0034] In this experiment, MCF-7 estrogen-dependent breast cancer cells were used as the biological test carrier, and the estrogen-like activity was determined by the classic E-SCREEN method.
[0035] In this experiment, DMSO solvent control group (negative), 1nM estradiol control group (positive), red cloverin group (1uM, 10uM) and peraxanthin group (1uM, 10uM) were set.
[0036] The results show that: red cloverin and prunus can significantly promote the proliferation of MCF-7 cells, and have estrogen-like effects, but their activity is only 10 times that of estradiol. -5 -10 -3 .
Embodiment 2
[0038] Molecular simulation of the interaction between pratensein and prunetin and estrogen receptor
[0039] Using computer-aided drug design method, using estrogen receptor (ER β, ER α) as the target model, the active conformation of pratensein and prunetin molecules was obtained by molecular mechanics optimization, and the molecular docking program was used to calculate the relationship between the receptor and the ligand. interaction.
[0040] The results showed that both pratensein and prunetin could enter the binding cavity of estrogen receptor, and the results also indicated that the binding force of both pratensein and prunetin was 7-10 times stronger than that of ER α. In addition, some positions on the molecule are substituted, which has a certain influence on its activity.
[0041] Due to the differences in tissue distribution of human ER β and ER α, ER β mainly exists in the brain, bone, bladder and vascular epithelial tissues, suggesting that pratensein and prune...
Embodiment 3
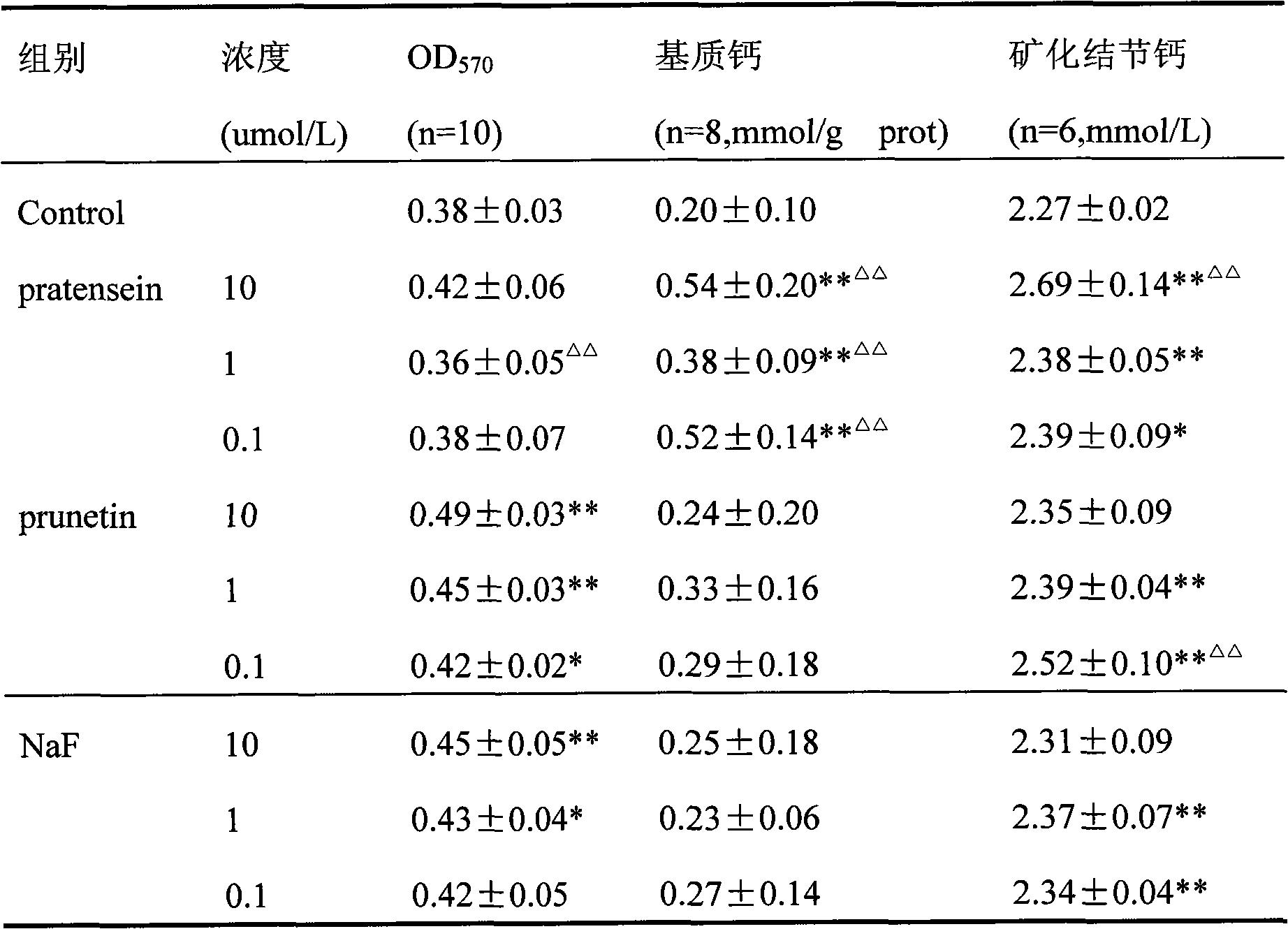
[0043] Effects of pratensein and prunetin on osteoblasts cultured in vitro
[0044] Separation of osteoblasts from newborn 1-3 day old SD rat skulls by enzymatic digestion, the third generation cells were observed by MTT method and methylthymol blue (MTB) colorimetric method to observe the effect of red cloverin and prunin on in vitro culture. Effects of osteoblast proliferation, cellular matrix calcium, and mineralized nodular calcium.
[0045] The results showed that: red cloverin and prunus at concentrations of 0.1-10umol / L can significantly promote the proliferation of osteoblasts, and can significantly increase the calcium in the cell matrix and calcium in mineralized nodules. The specific results are shown in the table below:
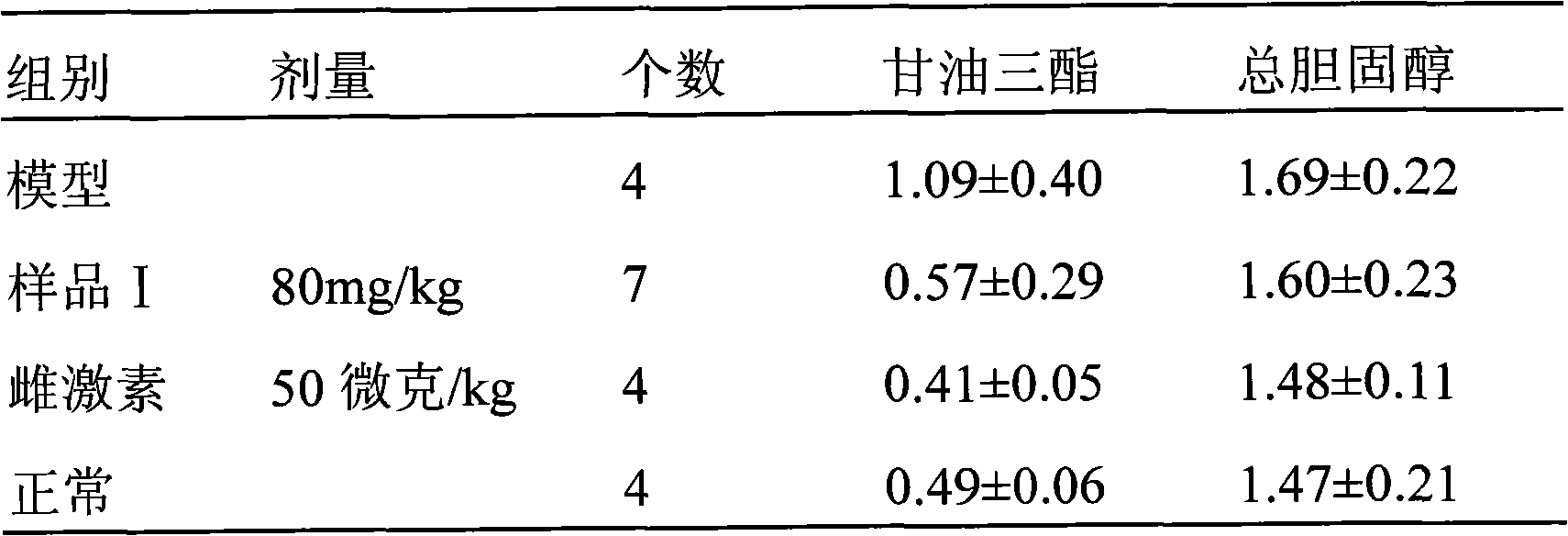
[0046]
[0047] *P△ P△△ P<0.01 compared with NaF
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com