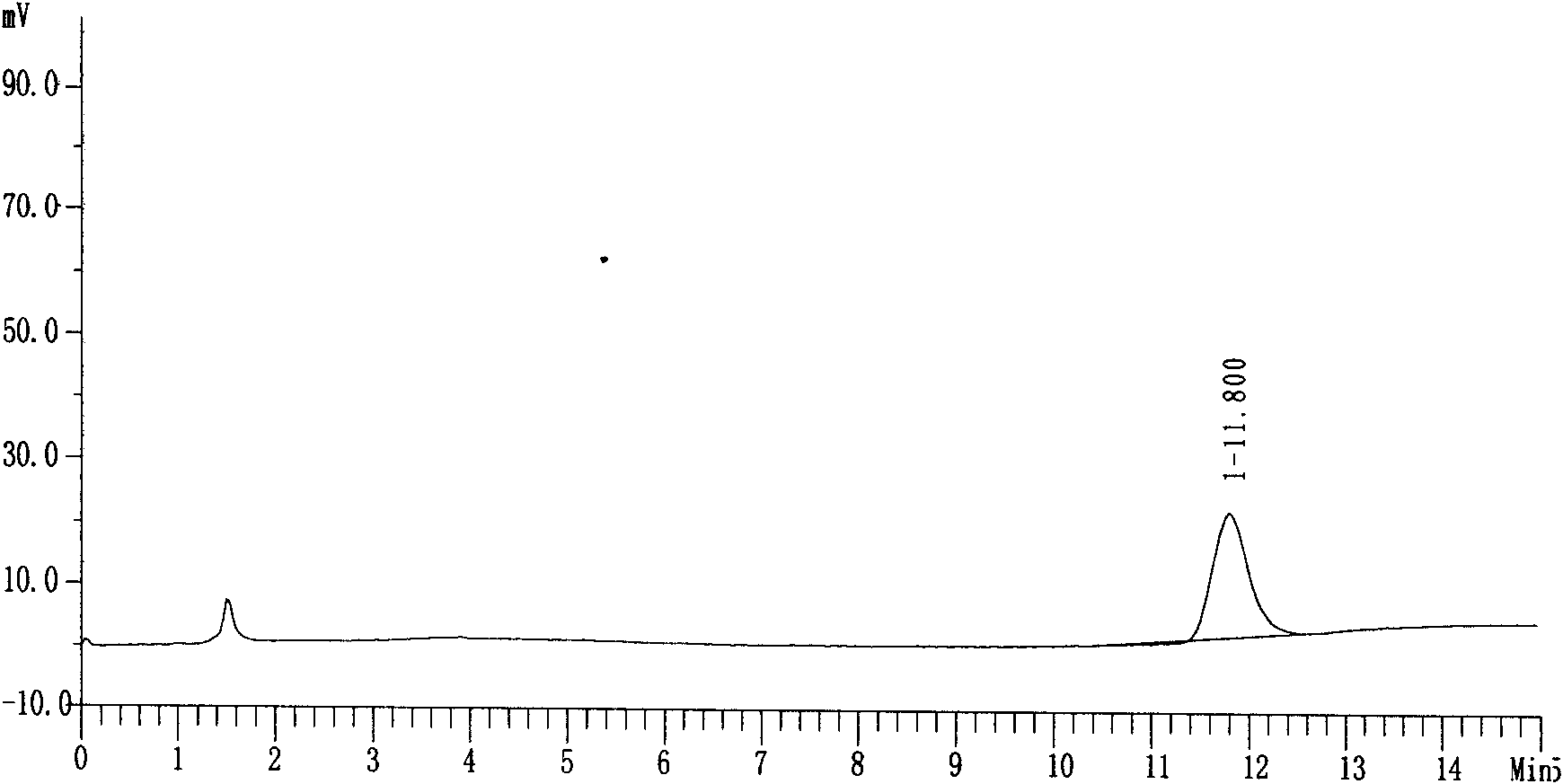
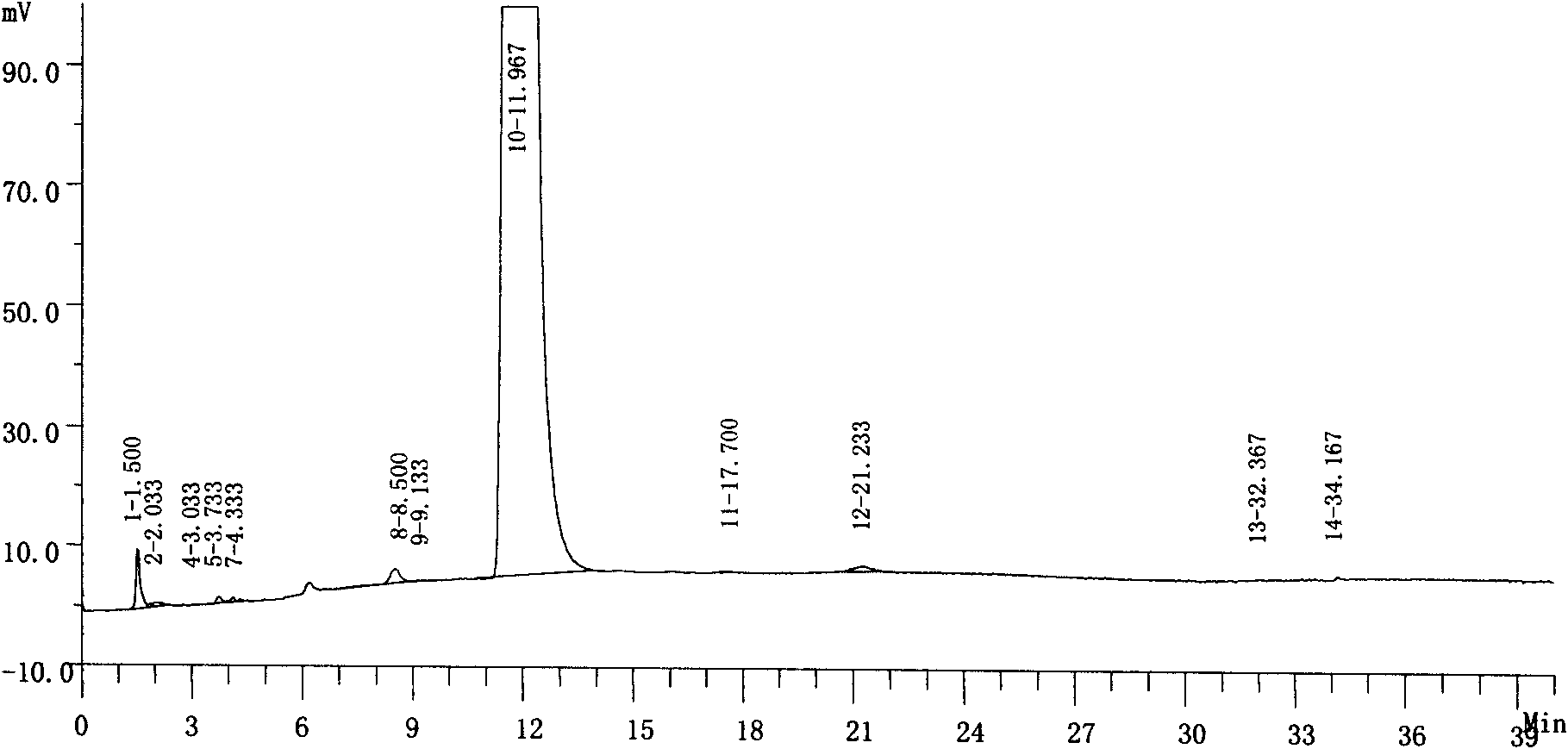
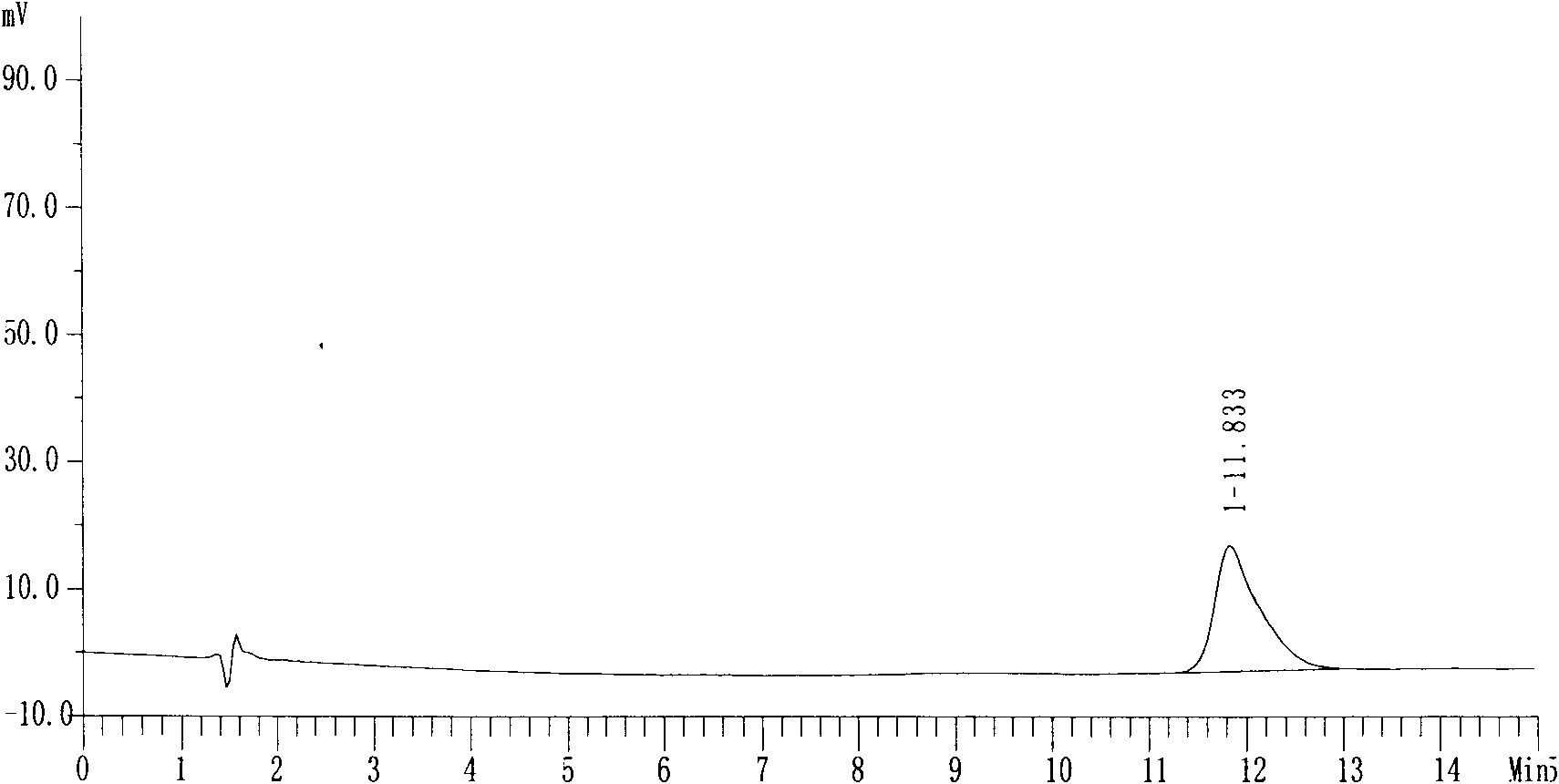
Preparation method of nifuratel with high purity
A technology for nifuratel and crude nifuratel, which is applied in the field of preparation of nifuratel, can solve the problems of low reaction yield and achieve the effect of high purity and good appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 5-nitrofurfural
[0027] Into a 20L reactor, add 7.15kg of isopropanol, 2.20kg of 5-nitrofurfural diethyl ester, 4.97kg of dilute nitric acid (mixed solution of 0.77kg of concentrated nitric acid and 4.20kg of distilled water), and heat to reflux for 2h. After cooling down to room temperature, the solution was kept for later use, and it was directly used in the next reaction.
Embodiment 2
[0029] Preparation of 5-nitrofurfural
[0030] In a 20L reactor, add 7.15kg of ethanol, 2.20kg of 5-nitrofurfural diethyl ester, 4.97kg of dilute sulfuric acid (mixed solution of 0.77kg of concentrated sulfuric acid and 4.20kg of distilled water), and heat to reflux for 2h. After cooling down to room temperature, the solution was kept for later use, and it was directly used in the next reaction.
Embodiment 3
[0032] Preparation of 5-nitrofurfural
[0033] In a 20L reactor, add 7.15kg of ethanol, 2.20kg of 5-nitrofurfural diethyl ester, 4.97kg of dilute nitric acid (mixed solution of 0.77kg of concentrated nitric acid and 4.20kg of distilled water), and heat to reflux for 2h. After cooling down to room temperature, the solution was kept for later use, and it was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com