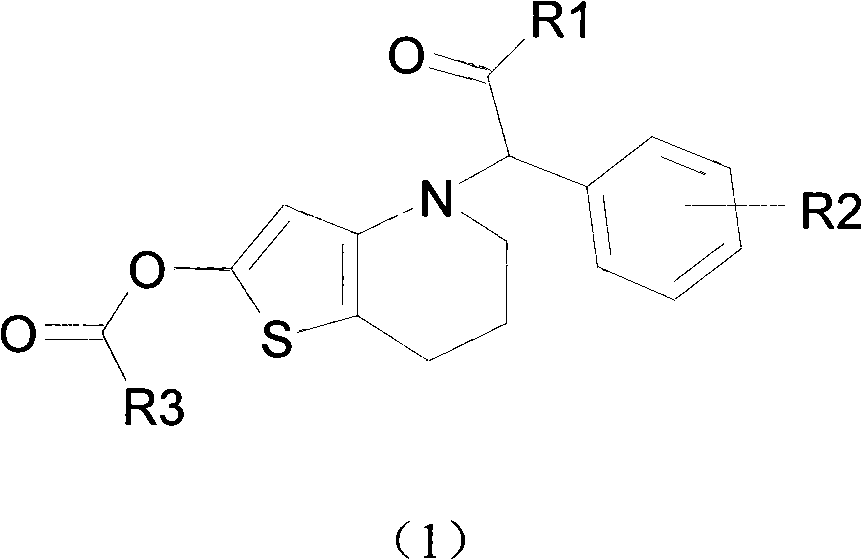
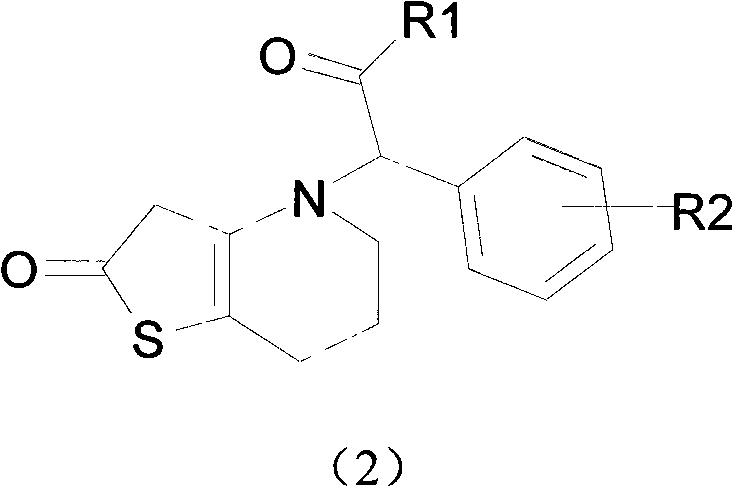
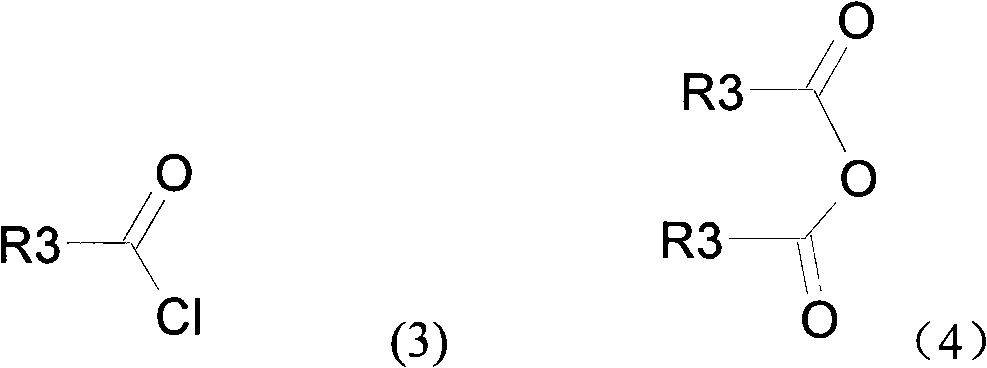
Novel compound with blood coagulation resisting function
A compound, C1-C6 technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 5-N-[(1RS)-2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothiophene[3,2-c Preparation of ]pyridin-2-yl acetylsalicylate
[0061]
[0062] Compound 1
[0063] 368 mg of 5-[(1RS)-2-cyclopropyl-1-(2-fluorophenyl 1)-2-oxoethyl]-4,5,6,7-tetrahydrothiophene[3,2 -c] Pyridin-2(4H)-one hydrochloride and 100mg of sodium hydride (52%, dispersed in mineral oil) were added to a 25ml reaction flask, then 2ml of anhydrous tetrahydrofuran was added, stirred for 30 minutes, and then added 200 mg of salicyloyl chloride was stirred and reacted for 2 hours, the reaction was stopped, and 57.9 mg of a yellowish solid was obtained by column chromatography, with a yield of 11.7%.
[0064] 1 H-NMR (400MHz, CDCl 3 ): δ8.11(d, 1H), 7.58(t, 1H), 7.28(m, 2H), 7.02-7.06(m, 2H), 6.85-6.91(m, 2H), 5.45(s, 1H), 4.77(s, 1H), 3.06(t, 2H), 2.55(t, 2H), 2.10(s, 3H), 1.86(m, 2H), 1.08(m, 1H), 0.55-0.77(m, 4H) .
[0065] ESI-MS: m / z 494 (MH + ).
Embodiment 2
[0067] S(+)-2-(2-chlorophenyl)-2-(2-acetylsalicylate-4,5,6,7-tetrahydrothiophene[3,2-c]pyridine)-5 - Preparation of methyl acetate
[0068]
[0069] Compound 2
[0070] 337 mg of S(+)-2-(2-chlorophenyl)-2-(4,5,6,7-tetrahydrothiophene[3,2-c]pyridin-2(4H)-one-5 - Add methyl acetate and 100mg of sodium hydride (52%, dispersed in mineral oil) to a 25ml reaction flask, then add 2ml of anhydrous tetrahydrofuran, stir for 30 minutes, then add 200mg of salicyloyl chloride, continue stirring for 2 hours , the reaction was stopped, and 61.2 mg of off-white solid was obtained by separation by column chromatography, with a yield of 12.2%.
[0071] 1 H-NMR (400MHz, CDCl 3 ): δ8.10(d, 1H), 7.55(t, 1H), 7.26(m, 2H), 7.13(m, 1H), 7.00-7.06(m, 3H), 6.85-6.91(m, 2H), 5.45(s, 1H), 4.77(s, 1H), 3.68(s, 3H), 3.06(t, 2H), 2.55(t, 2H), 2.10(s, 3H), 1.86(m, 2H).
[0072] ESI-MS: m / z 500 (MH + ).
Embodiment 3
[0074] S(+)-2-(2-chlorophenyl)-2-nicotinate-4,5,6,7-tetrahydrothiophene[3,2-c]pyridine)-5-methyl acetate preparation
[0075]
[0076] Compound 3
[0077] 337 mg of S(+)-2-(2-chlorophenyl)-2-(4,5,6,7-tetrahydrothiophene[3,2-c]pyridin-2(4H)-one-5 - Methyl acetate and 100mg of sodium hydride (52%, dispersed in mineral oil) were added to a 25ml reaction flask, then 2ml of anhydrous tetrahydrofuran was added, after stirring for 30 minutes, 150mg of nicotinoyl chloride was added, and the reaction was continued for 2 hours, The reaction was stopped, and 75.6 mg of off-white solid was separated by column chromatography, with a yield of 17.1%.
[0078] 1 H-NMR (400MHz, CDCl 3 ): δ9.12(s, 1H), 8.79(d, 1H), 8.17(d, 1H), 7.49(t, 1H), 7.15(m, 1H), 7.02-7.06(m, 3H), 6.85- 6.91 (m, 2H), 5.48 (s, 1H), 4.75 (s, 1H), 3.67 (s, 3H), 3.06 (t, 2H), 2.53 (t, 2H), 1.83 (m, 2H).
[0079] ESI-MS: m / z 443 (MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com