Oxime compound, preparation process and application thereof
A technology of compound and preparation method, applied in the field of oxime compounds and their preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
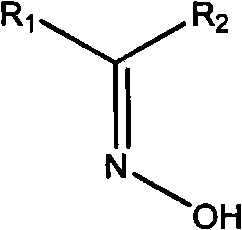
[0019] Example 1: Preparation of (E)-2-(4-bromophenyl)-1-(3,4-dihydroxyphenyl)ethanone oxime (compound 1)
[0020]
[0021] Add catechol (10mmol) and p-bromophenylacetic acid (10mmol) into a 100ml single-necked round bottom flask, and then add 10ml of boron trifluoride ether (47.0-47.7%, calculated as BF3, the same below). Stir the reaction, heat to 80-84°C and reflux for about 2 hours, then cool, add sodium acetate (8g) to a water (200ml) solution, stir while adding, then stand still, filter after 24h to obtain a brown solid. Put the obtained product into a 100ml beaker, add absolute ethanol to dissolve completely, then filter after recrystallization to obtain yellow crystalline substance 2-(4-bromophenyl)-1-(3,4-dihydroxy) Phenyl ethyl ketone. Then move it into a 100ml single-necked round bottom flask, add sodium acetate and hydroxylamine hydrochloride of equimolar mass, dissolve in ethanol (10ml). Stir and heat to 60°C, reflux for about 3 hours, then detect the reactio...
Embodiment 2
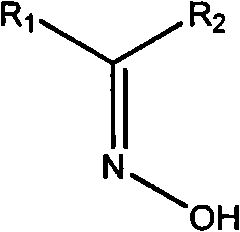
[0022] Example 2: Preparation of (E)-2-(4-bromophenyl)-1-(2,3,4-hydroxyphenyl)ethanone oxime (compound 2)
[0023]
[0024] The preparation method is the same as in Example 1. Pyrogallol was used instead of catechol to obtain the target compound in brown granular form. Yield 63%. Mp 143-1145°C. 1 HNMR (300MHz, DMSO-d6), 4.14(s, 2H), 6.24(s, 1H), 6.39(d, J=9Hz, 1H), 6.90(d, J=8Hz, 2H), 7.08(d, J =9Hz, 2H), 7.88(d, J=9Hz, 1H), 8.71(s, 1H), 9.45(s, 1H), 10.11(s, 1H), 12.45(s, 1H).ESI-MS: 336.99 ([M+H]+).Anal.Calcd for C14H12BrNO4: C, 49.73; H, 3.58; Br, 23.63; N, 4.14; O, 18.93%; found: C, 49.71; H, 3.56; N, 4.15; O, 18.97%.
Embodiment 3
[0025] Example 3: Preparation of (E)-2-(4-methoxyphenyl)-1-(2,4,6-hydroxyphenyl)ethanone oxime (compound 3)
[0026]
[0027] The preparation method is the same as in Example 1. Pyroglucinol was used instead of catechol, and p-methoxyphenylacetic acid was used instead of p-bromophenylacetic acid to obtain the brown powdery target compound. Yield 60%. Mp 143-144°C. 1 H NMR (300MHz, DMSO-d6), 3.93(s, 2H), 6.37(d, J=9Hz, 1H), 6.92(d, J=8Hz, 2H), 7.15(d, J=8Hz, 2H), 7.34(d, J=9Hz, 1H), 9.15(s, 1H), 10.34(s, 1H), 12.11(s, 2H), 12.98(s, 1H). ESI-MS: 289.10([M+H] +).Anal.Calcd for C15H15NO5: C, 62.28; H, 5.23; N, 4.84; O, 27.65%; found: C, 62.24; H, 5.22; N, 4.85; O, 27.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com