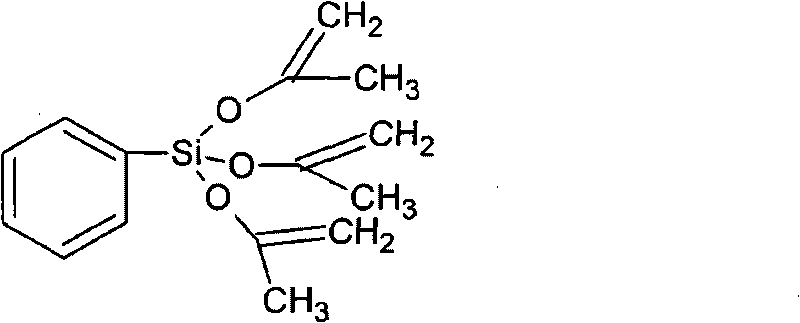
Method for preparing phenyl iso-propenyloxysilane
A technology of phenylisopropenyloxysilane and phenyltrichlorosilane, which is applied in the field of preparation of phenylisopropenoxysilane, can solve the problems of research or production inconvenience, poor selectivity, and many by-products, and achieve the price of raw materials Inexpensive, easy to obtain raw materials, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a three-necked flask containing 500g of cyclohexane and 580g (10 moles of acetone), 8g of catalyst sodium carbonate was added under stirring, and 212g (1.0 moles) of phenyltrichlorosilane was added dropwise at 60°C for 3 hours. , keep warm for 5 hours. It was cooled and filtered to obtain 1152.5 g of filtrate. The composition of the filtrate was analyzed by a gas chromatograph (GC-2014, available from Shimadzu Corporation), and it was found that the filtrate was a mixture of 43.36% by weight of cyclohexane and 22% by weight of phenyltriisopropenyloxysilane.
[0020] The filtrate obtained as above was subjected to vacuum distillation, and 257 g of 150-170°C / 2-5kPa fraction was collected, and measured by gas chromatograph (GC-2014, purchased from Shimadzu Company), phenyltriisopropenyloxysilane in the fraction was The purity is 99.0145% by weight. The yield of phenyltriisopropenyloxysilane product was 92.03%.
Embodiment 2
[0022] In a three-necked flask containing 500 g of diethyl carbonate and 348 g of acetone (6 moles), 15 g of catalyst calcium carbonate was added under stirring, and 212 g of phenyltrichlorosilane (1.0 moles) was added dropwise at 120° C. The addition time was 3 hours, keep warm for 7 hours. It was cooled and filtered to obtain 938 g of filtrate. The composition of the filtrate was analyzed by a gas chromatograph (GC-2014, available from Shimadzu Corporation), and it was found that the filtrate was a mixture of 42.66% by weight of diethyl carbonate and 25.2% by weight of phenyltriisopropenyloxysilane.
[0023] The filtrate obtained as above is subjected to vacuum distillation, and 232 g of 150-170°C / 2-5kPa fractions are collected and measured with a gas chromatograph (GC-2014, purchased from Shimadzu Company), phenyltriisopropenyloxysilane in the fractions The purity is 98.7532% by weight. The yield of phenyltriisopropenyloxysilane product was 82.86%.
Embodiment 3
[0025] In a three-necked flask containing 500g of a mixed solvent of dimethyl carbonate and acetonitrile and 174g (3 moles) of acetone, 10g of catalyst potassium carbonate was added under stirring, and 212g (1.0 moles) of phenyltrichlorosilane was added dropwise at 60°C, The dropwise addition time was 3 hours, and the temperature was kept for 5 hours. It was cooled and filtered to obtain 765 g of filtrate. The composition of the filtrate was analyzed with a gas chromatograph (GC-2014, available from Shimadzu Corporation), and it was found that the filtrate was a mixture of 42.0% by weight of diethyl carbonate and 36.2% by weight of phenyltriisopropenyloxysilane.
[0026] The filtrate obtained as above is subjected to vacuum distillation, and 267 g of 150-170°C / 2-5kPa fractions are collected, and measured by gas chromatograph (GC-2014, purchased from Shimadzu Company), phenyltriisopropenyloxysilane in the fractions The purity is 99.2384% by weight. The yield of phenyltriisopr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com