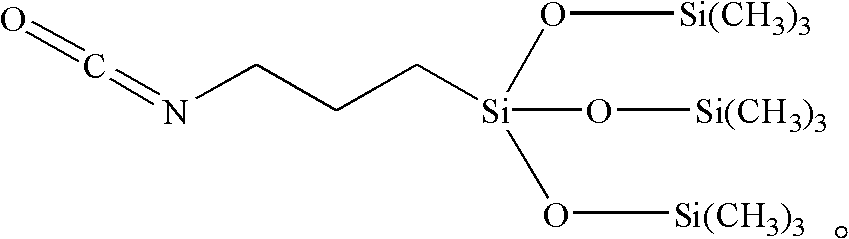
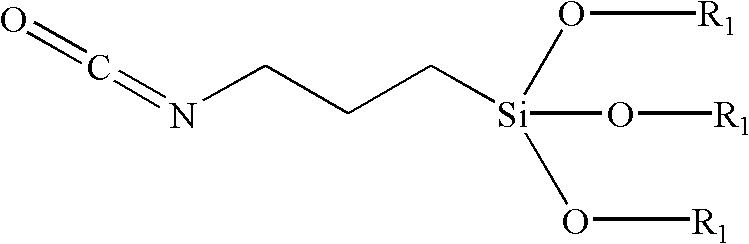
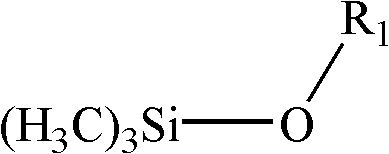Preparation method for 3- isocyanate propyl (trimethylsilanolate) silane
A technology of ester group propyltrialkoxysilane and trimethylsiloxy, which is applied in the field of organic synthesis, can solve problems such as unseen technical routes and reports, and achieve the effect of simple process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a three-necked flask containing 207.0 g (1.0 mol) of isocyanatopropyl trimethoxysilane and 486.0 g (3.0 mol) of hexamethyldisiloxane, add concentrated sulfuric acid (20.8 g, 3%) and stir to 60~62℃, react at 60~62℃ for 12 hours, then gradually increase the temperature to 120±2℃, and react at a constant temperature of 120±2℃. During the reaction process, the trimethylmethoxysilane is continuously extracted under normal pressure. In order to promote the reaction to the right, improve the reaction efficiency and shorten the reaction time; take a sample after a constant temperature of 120±2°C for 6 hours, and detect it by gas chromatography GC-2014 (Island Feng), when the content of isocyanatopropyltrimethoxysilane in the reaction mixture When the content of isocyanatopropylmethoxy bis(trimethylsiloxy)silane is less than 1% (about 8h), the reaction is over; the reaction mixture is cooled at 30-60°C and the pressure is 2-10KPa Remove residual trimethylmethoxysilane and unr...
Embodiment 2
[0026] In a three-necked flask containing 249.0 g (1.0 mol) of isocyanatopropyl triethoxysilane and 486.0 g (3.0 mol) of hexamethyldisiloxane, add trifluoromethanesulfonic acid (0.735 g, 0.1%) Raise the temperature to 80°C under stirring, and react at 80±2°C for 24 hours, then gradually increase the temperature to 120±2°C, and react at a constant temperature of 120±2°C. Sampling after constant temperature 6h, detect by gas chromatography GC-2014 (Island Feng), when the content of isocyanate propyl triethoxysilane in the reaction mixture is less than 0.2%, and isocyanate propyl ethoxy di( When the content of trimethylsilyl)silane is less than 1% (about 10h), stop the reaction; cool the resulting mixture, and remove the residual trimethylethoxy silicon in the mixture at 60-100°C and a pressure of 10-30KPa and unreacted hexamethyldisiloxane to obtain a mixture containing isocyanatopropyl tris(trimethylsiloxy)silane with a content of 85.7835%, 421.9 g.
[0027] The above mixture ...
Embodiment 3
[0029] In a three-necked flask containing 207.0 g (1.0 mol) of isocyanatopropyl trimethoxysilane and 1620.0 g (10.0 mol) of hexamethyldisiloxane, add trifluoromethanesulfonic acid (18.27 g, 1%) and stir Raise the temperature to 60°C under low temperature, and react at 60-62°C for 8 hours, gradually increase the temperature to 120±2°C, and react at a constant temperature of 120±2°C. Silane; sampling after constant temperature 6h, detected by gas chromatography GC-2014 (Island Feng), when the content of isocyanate propyl trimethylsilane in the reaction mixture is less than 0.2%, and isocyanate propyl methoxy bis (trimethylsilyl) When the silane content is less than 1% (about 10h), stop the reaction, cool the reaction mixture, and remove the residual trimethylethoxy silicon and unreacted Hexamethyldisiloxane, cooled to obtain a mixture containing isocyanatopropyl tris(trimethylsiloxy)silane, content 89.6142wt%, 413.9g.
[0030] The mixture obtained above was subjected to vacuum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com