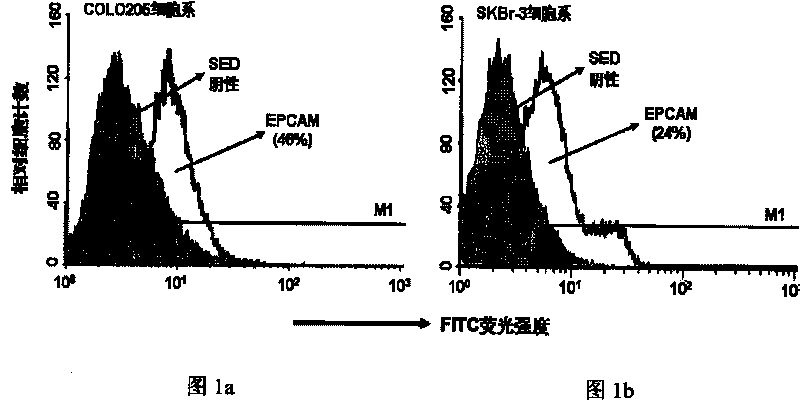
Variable regions of light chains and heavy chains of FMU-EPCAM-2A9 monoclonal antibodies
A technology of FMU-EPCAM-2A9, 1. FMU-EPCAM-2A9, applied in the field of monoclonal antibodies, can solve the problems of antibody drug hypersensitivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The applicant immunized BALB / c mice with recombinant human EPCAM, prepared a group of mouse anti-human EPCAM monoclonal antibodies, and screened a hybridoma cell line that can stably secrete FMU-EPCAM-2A9 monoclonal antibody with high affinity against human EPCAM , Prepare ascites fluid to obtain high-affinity anti-human EPCAM monoclonal antibody. Confirm the uniqueness of the gene sequence and corresponding protein sequence and its CDR sequence; provide support for anti-human EPCAM chimeric or humanized genetic engineering antibodies.
[0032] The present invention will be described in detail below in conjunction with the accompanying drawings, which are explanations of the present invention rather than limitations.
[0033] The present invention is specifically implemented according to the following steps:
[0034] 1 Preparation of mouse anti-human EPCAM high-affinity antibody
[0035] 1.1 Preparation and purification of monoclonal antibodies
[0036] Design primer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com