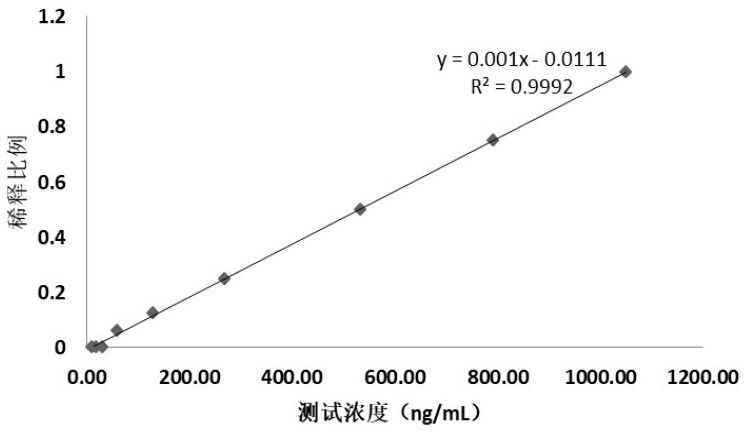
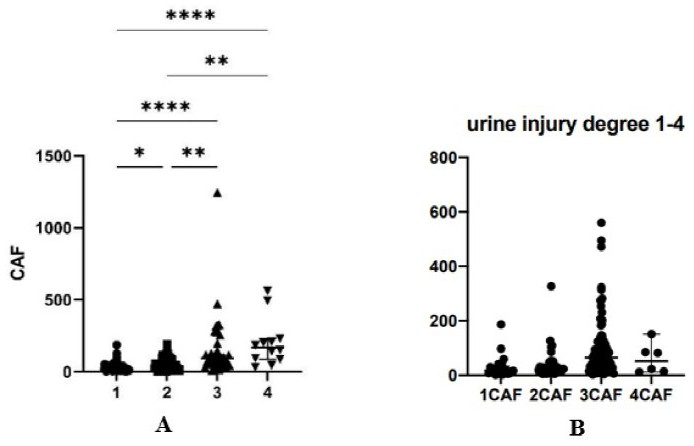
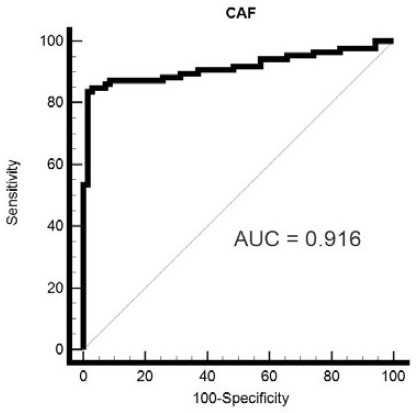
Application of CAF22 detection reagent in preparation of composition for evaluating renal tubular injury
A kidney tubule and composition technology, applied in the field of biological diagnosis, can solve the problems of poor diagnostic specificity of renal tubule injury, loss of treatment time, and inability to confirm abnormal renal function, etc., and achieve the effect of multi-possibility, improved sensitivity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Sample Collection
A total of 157 urine samples with abnormal renal status were collected.
[0035] The abnormal renal status is manifested as: abnormal albumin, immunoglobulin G, transferrin, α1 microglobulin, lysosomal enzymes, and creatinine indexes in urine.
Embodiment 2
[0036] Embodiment 2, the preparation method of CAF22 chemiluminescence detection kit
The CAF22 chemiluminescence detection kit of the present invention mainly includes a conjugate-labeled CAF22 antibody, a chemiluminescence label-labeled CAF22 antibody, streptavidin magnetic particles, a chemiluminescence substrate, a CAF22 standard and a quality control product.
[0037] The preparation method of the CAF22 chemiluminescence detection kit of the present invention mainly comprises the following steps:
Step 1) Preparation of conjugate-labeled CAF22 antibody
The activator biotin ester was added to the CAF22 antibody after desalting in a molar ratio of 1:10, and the reaction was performed at room temperature for 4 hours in the dark. The reacted solution was added to the pre-rinsed PD-10 purification column for purification. The purified conjugate was measured with A280 and stored at -20°C after adding an equal volume of glycerol. When in use, dilute to a concentration of 0.1...
Embodiment 4
[0046] Example 4. Method for detecting CAF22 with CAF22 chemiluminescence detection kit
The chemiluminescence detection kit for determining CAF22 of the present invention includes reagent R1, reagent R2, streptavidin magnetic beads, chemiluminescence substrate, CAF22 calibrator and quality control substance.
[0047] R1 is a biotin-conjugated CAF22 antibody, stored in 50 mM PBS pH 7.4 and 0.15 M NaCl, the buffer also contains 0.1% sodium azide, 0.5 g / L Tween 20 and 1 g / L bovine serum albumin.
[0048]R2 is an alkaline phosphatase-labeled antibody to CAF22, maintained in 0.05 M PBS and 0.15 M NaCl in a buffer also containing 0.1% sodium azide, 0.5 g / L Tween 20, and 1 g / L sodium caseinate.
[0049] The concentration of streptavidin magnetic beads is 1 mg / mL, and the particle size of the magnetic beads is 2.8 μM.
[0050] The chemiluminescent substrate is APS-5.
[0051] A fully automatic chemiluminescence immunoassay was used as the detection tool, the detection mode was san...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com