Azithromycin injection and preparation method thereof
A technology of azithromycin and injection, applied in the field of biomedicine, can solve the problems of cumbersome operation, poor stability, and easy side effects, etc., and achieve the effect of enhanced solubility and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
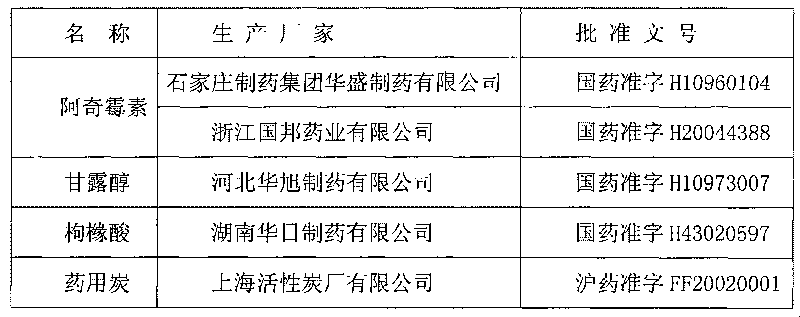
[0090] Embodiment one: a kind of specification is 0.25g (250,000 units) azithromycin injection, in the injection of 3000ml, contains following each composition: the azithromycin (anhydrous substance) of 250.0g, the citric acid of 1.0g, 120.0g mannitol, 18.0g of medicinal charcoal, and the balance is fresh water for injection.
[0091] The preparation method of above-mentioned azithromycin injection, its concrete steps are as follows:
[0092] ①. Add mannitol to fresh water for injection with a volume of 10%, heat it to dissolve completely, add medicinal charcoal with half the amount of the formula, boil for 30 minutes, decarbonize and filter;
[0093] ②. Add the formulated amount of azithromycin to the water for injection below 20°C with a volume of 60% to make a suspension;
[0094] ③. Dissolve citric acid with an appropriate amount of water for injection and slowly add it to the azithromycin suspension. Stir while adding to completely dissolve the azithromycin. Strictly con...
Embodiment 2
[0099] Embodiment two: a kind of specification is 0.5g (500,000 units) azithromycin injection, in the injection of 3000ml, contains following each composition: the azithromycin of 500.0g, the citric acid of 1.0g, the mannitol of 120.0g, 18.0g g of medicinal charcoal, and the balance is fresh water for injection.
[0100] The preparation method of above-mentioned azithromycin injection, its concrete steps are as follows:
[0101] ①. Add mannitol to fresh water for injection with a volume of 10%, heat it to dissolve completely, add medicinal charcoal with half the amount of the formula, boil for 30 minutes, decarbonize and filter;
[0102] ②. Add the formulated amount of azithromycin to the water for injection below 20°C with a volume of 60% to make a suspension;
[0103] ③. Dissolve citric acid with an appropriate amount of water for injection and slowly add it to the azithromycin suspension. Stir while adding to completely dissolve the azithromycin. Strictly control the pH va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com