Monoclonal antibody for resisting Cyr61 protein and application thereof
A monoclonal antibody and protein technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-inflammatory agents, etc., can solve the problem of high technical difficulty of monoclonal antibodies and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
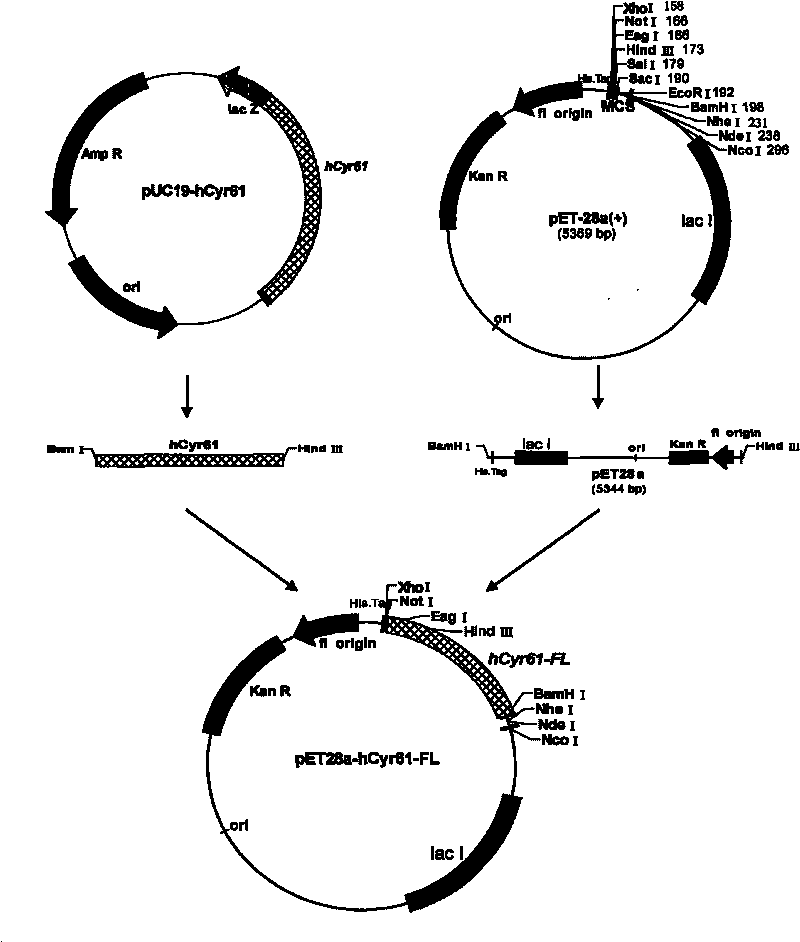
[0046] Example 1 Constructing and expressing human Cyr61 full-length (26-381) protein plasmid and protein expression
[0047] According to the principles of primer design and the 2295 bp full-length cDNA sequence of human Cyr61 published in GeneBank (NM 001554), the primer design software Primer 5.0 was used to design, remove the signal peptide part, and add corresponding restriction enzyme sites at both ends.
[0048] Primers for Cyr61 full-length (26-381) protein:
[0049] Upstream primer: 5'-TT GGATCC TGCCCCGCTGCCTGCCACT-3'
[0050] (the underline is the BamH I restriction site);
[0051] Downstream primer: 5'-TT AAGCTT CTAGTCCCCTAAATTTGTGAATGTCATT-3'
[0052] (The underline is the Hind III restriction site).
[0053] Engineering bacteria: BL21(DE3), expression vector: pET28(a).
[0054] The plasmid pET28(a) was co-digested with restriction endonucleases BamH I and Hind III respectively, and the amplified human full-length Cyr61 (hCyr61-FL) was ligated into the pET2...
Embodiment 2
[0058] Example 2 Constructing and expressing human Cyr61 full-length (103-381) protein plasmid and protein expression
[0059] Primer for the C-terminal fragment of Cyr61 protein (Cyr61-XC-103-381aa)
[0060] Upstream primer: 5'-TT GGATCC GGCTGTCCCAACCCTCGGCTG-3'
[0061] (the underline is the BamH I restriction site);
[0062] Downstream primer: 5'-TT AAGCTT CTAGTCCCCTAAATTTGTGAATGTCATT-3'
[0063] (The underline is the Hind III restriction site);
[0064] Engineering bacteria: BL21(DE3), expression vector: pET28(a).
[0065] Plasmid pET28(a) was co-digested with restriction endonucleases BamH I and Hind III respectively, and the amplified human Cyr61 near C-terminal 2 / 3 fragment (hCyr61-XC-103-381aa) was respectively Insert into the pET28(a) vector to obtain the corresponding recombinant expression plasmid: pET28a-hCyr61-XC (see Figure 5 ).
[0066] Identification of the recombinant plasmid of the His-Cyr61 C-terminal fragment: Extract the total RNA of synoviocyte...
Embodiment 3
[0069] Example 3 Preparation of mouse anti-human Cyr61 monoclonal antibody and analysis of binding sites
[0070] BABC / c mice: female, 4 mice aged 6-8 weeks and 50 mice aged 8-10 weeks, provided by the Animal Center of Shanghai Academy of Sciences, Chinese Academy of Sciences.
[0071] Mouse myeloma cells SP20 / Ag-14, preserved for passage.
[0072] It was prepared according to the conventional monoclonal antibody preparation method, and the Cyr61 protein used for identification was a commercially available recombinant human Cyr61 protein (PeproTech Inc. New Jersey, USA, Cat: 120-25).
[0073] According to conventional immunization methods, BABL / c mice were immunized with self-made human His-hCyr61 fusion protein, and mouse splenocytes were collected, and cell fusion was carried out according to classical methods. After 5 consecutive subcloning and ELISA screening, 9 hybridoma cells can stably secrete specific anti-human Cyr61 antibody (only react with purified or His-hCyr61, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com