Entecavir injection
A technology of entecavir injection and entecavir, applied in the field of entecavir injection and its preparation, can solve the problems of malabsorption, delayed absorption of entecavir, decreased bioavailability, etc., and achieves good inhibitory effect and good stability.
Inactive Publication Date: 2010-06-02
沈阳双鼎制药有限公司
View PDF5 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, the pharmaceutical preparations of entecavir marketed at home and abroad are all oral dosage forms, such as tablets and oral liquids. Eating will cause delays in the absorption of entecavir, which will lead to malabsorption and decreased bioavailability. Therefore, such oral dosage forms should be taken on an empty stomach, at least Take it two hours before or after meals to achieve better drug absorption
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
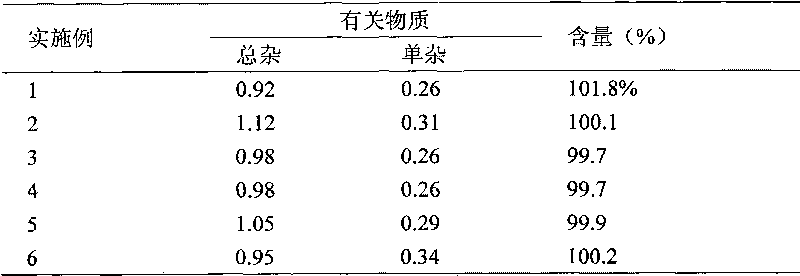
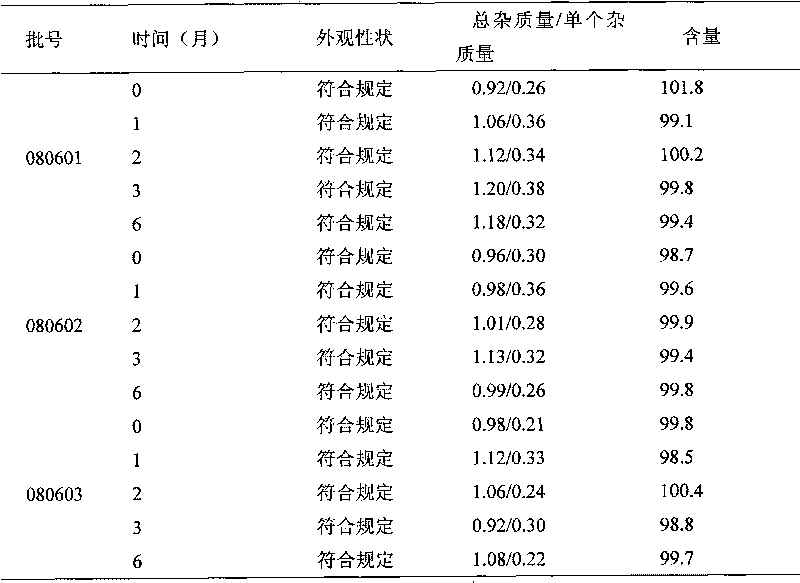
Embodiment 1
[0043] Entecavir 5mg
[0045] Add water for injection to 1000mL
Embodiment 2
[0047] Enteca 0.1g
[0048] Propylene glycol 2mL
[0050] Add water for injection to 1000mL
Embodiment 3
[0052] Entecavir 1.0g
[0053] Propylene glycol 10mL
[0055] Add water for injection to 1000mL
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to an Entecavir injection comprising a small-capacity injection, a large-capacity sterilized injection and a freeze-dry sterile powder injection. The Entecavir injection is characterized by being prepared from 0.1-2.5mg of single-dosage active Entecavir and an additive which comprises a solvent for injection, 0-10% of pH regulator, 0-10% of antioxidant, 0-60% of solubilizer, 0-20% of isotonic regulator, 0.1-20% of filler and 0.1-20% of filling scaffold agent, and the percentage is the percent of the total quality of the injection in the liquid volume. The Entecavir comprises the injection and the sterile powder injection and has favorable inhibited effect on hepatitis B and good stability.
Description
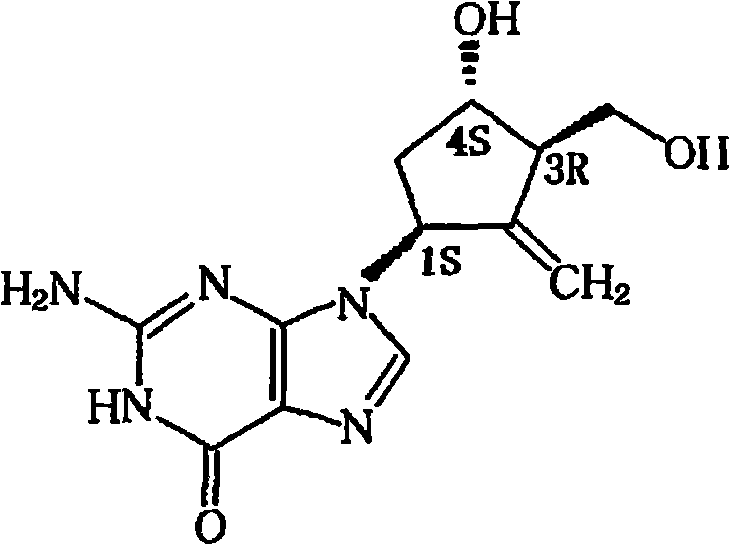
technical field [0001] The invention relates to an injection of entecavir and a preparation method thereof, specifically, a sterilized solution, emulsion or suspension for injection into the body made of active form of entecavir, as well as a preparation before use Sterile powder or concentrated solution for solution or suspension. Background technique [0002] Entecavir is a guanosine analogue with the chemical name 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-hydroxymethyl-2-methylenecyclopentyl 】-1,9-dihydro-6H-purin-6-one, the molecular structure is as follows: [0003] [0004] Molecular formula C 12 h 15 N 5 o 3 , molecular weight 277.3, usually contains a crystal water, its monohydrate formula C 12 h 15 N 5 o 3 .H 2 O, molecular weight 295.3. [0005] US Patent No. 5,206,244 discloses entecavir and its use in the treatment of hepatitis B. International publications WO98 / 09964 and WO2004, 052310 respectively disclose two improved entecavir synthesis methods. US pa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/08A61K9/10A61K9/19A61K31/522A61P31/22A61P1/16
Inventor 马占芝李亚玲丁百莲
Owner 沈阳双鼎制药有限公司
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com