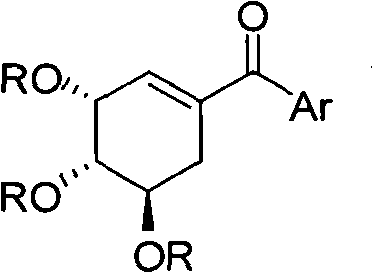
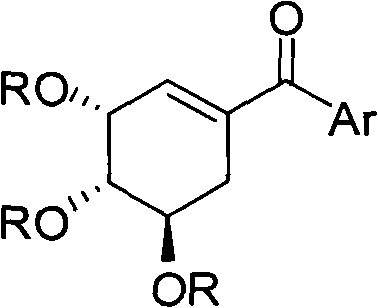
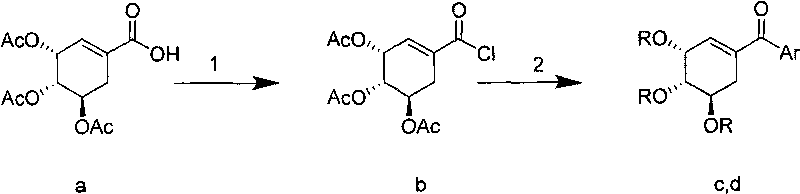
Shikimic acid compound and preparation method and application thereof
A compound, shikiki technology, applied in a class of shikimic acid compounds and its preparation and use, can solve problems such as inability to pass through the blood-brain barrier, limited application, and difficult absorption after oral administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 (compound c1):
[0028]
[0029] Add 500 mg of triacetylshikimic acid a (Bi Yi et al., Journal of China Pharmaceutical University, 2007, 38(2), 108-111). Dissolve in 5 mL of thionyl chloride, reflux for 2 hours, and spin dry under reduced pressure to obtain 525 mg of crude triacetylshikimoyl chloride (compound b), which can be directly used in the next reaction.
[0030] Add 70mg Pd(PPh 3 ) 4 , 665mgK 3 PO 4 ·3H 2 O, 183mg PhB(OH) 2 (phenylboronic acid) and 5mL anhydrous toluene, N 2 Reaction at 80°C for 8h under protection. After the reaction liquid was cooled, it was poured into water, extracted three times with ethyl acetate, the organic phases were combined and dried, spin-dried, and separated by petroleum ether: ethyl acetate = 4:1 silica gel column chromatography to obtain 390 mg of the target compound. 1 HNMR: 87.69-7.66 (m, 2H), 7.58-7.52 (m, 1H), 7.47-7.42 (m, 2H), 6.30-6.28 (m, 1H), 5.82-5.79 (m, 1H), 5.42-5.35 (m, 1H), 5.32-5.28 (m, 1...
Embodiment 2
[0031] Embodiment 2 (compound c2)
[0032]
[0033] The preparation process is the same as in Example 1, except that phenylboronic acid is replaced by p-tolylboronic acid. 1 HNMR: δ7.62-7.59(d, 2H, J=7.8), 7.26-7.23(d, 2H, J=7.8), 6.27-6.26(t, 1H, J=1.8), 5.80(s, 1H), 5.40-5.35 (m, 1H), 5.32-5.28 (m, 1H), 3.15-3.07 (dd, 1H, J=18.6, 4.8), 2.56-2.48 (dd, 1H, J=18.6, 4.8), 2.40 ( s, 3H), 2.08 (s, 3H), 2.06 (s, 6H). Yield: 72%.
Embodiment 3
[0034] Embodiment 3 (compound c3)
[0035]
[0036] The preparation process is the same as in Example 1, except that phenylboronic acid is replaced by p-methoxyphenylboronic acid. 1 HNMR: δ7.73-7.70(d, 2H, J=9), 6.94-6.90(d, 2H, J=9), 6.22-6.19(m, 1H), 5.80-5.77(t, 1H, J=3.9 ), 5.41-5.34(m, 1H), 5.30-5.26(m, 1H), 3.84(s, 3H), 3.13-3.05(dd, 1H, J=18.6, 5.7), 2.54-2.46(dd, 1H, J = 18.6, 6.3), 2.07 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H). Yield: 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com