Composition for externally detecting rheumatoid arthritis antibody and application thereof
An in vitro detection and composition technology, which is applied in the field of detecting the antigen composition of rheumatoid arthritis autoimmune antibodies and detecting the antigens of rheumatoid arthritis autoimmune antibodies, can solve the problem that there is no data to support BiP in predicting rheumatoid arthritis, Antibody differences, detection sensitivity can not be significantly improved and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] The synthesis of embodiment 1 polypeptide
[0141] Polypeptides are synthesized by solid-phase synthesis technology using Fmoc chemical method. For the specific steps of this method, see Eur.J.Immunol.1994, 24, 3188-3193; J.Org.Chem.1972, 37, 3404-3409; Huang Weide, Chen Changqing Polypeptide Synthesis, Beijing: Science Press, 1985.
[0142] The formation method and steps of disulfide bonds can be found in the literature: Huang Weide, Chen Changqing Peptide Synthesis, Beijing: Science Press, 1985, p85; Michael W.Pennington Peptide Synthesis Protocols (Methods in Molecular Biology), Humana Press, 1994, p91-169
[0143] Through the above steps, the specific sequence of the synthetic polypeptide is:
[0144] Polypeptide 1: His-Glu-Cys-His-Glu-Phe-Arg-Phe-Cit-Gly-Cit-Ser-Arg-Ala-Ala-Cys-Glu (SEQ ID No1), on which the third and tenth The six-position Cys forms a disulfide bond through the sulfhydryl group, and makes the polypeptide have a ring structure, and can simulate t...
Embodiment 2
[0146] The purification of embodiment 2 polypeptide
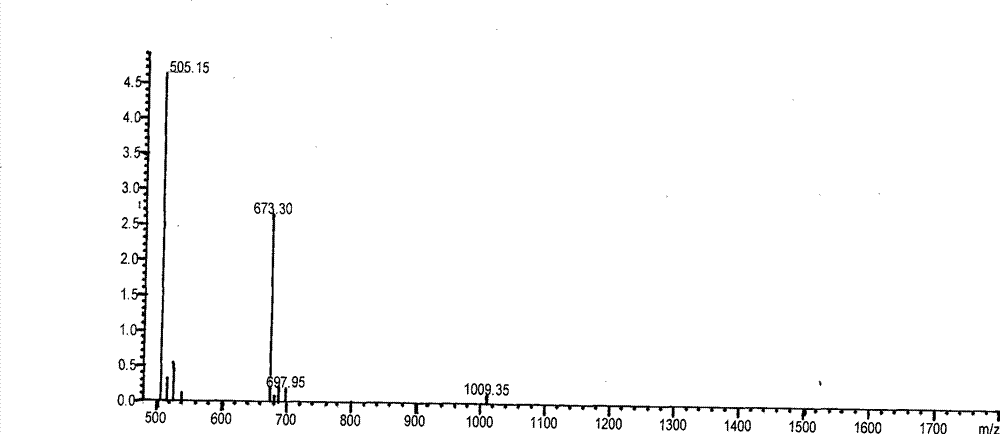
[0147] First, the protecting group at the end of the protein or polypeptide is deprotected and neutralized in dimethylamide (DMF) solvent. The polypeptide is cut off from the synthetic resin with hydrogen fluoride, and the obtained crude product is passed through a reversed-phase chromatography C18 or C8 column (such as: 5 μm, 250×4.6 mm), and the mobile phase A (0.1% (v / v) trifluoroacetic acid Acetonitrile solution) and mobile phase B (0.1% (v / v) trifluoroacetic acid in water) were gradient elution solvents. Within 45 minutes, the mobile phase A accounted for the total volume of the two phases A and B changed from 0% (v / v) to 100% (v / v) to collect the target polypeptide, and the organic phase was removed by desalting chromatographic column (GE Healthcare) or rotary evaporation. The solvent and the target polypeptide can be determined by combining LC-MS or directly injecting the collected polypeptide through the molecular ...
Embodiment 3
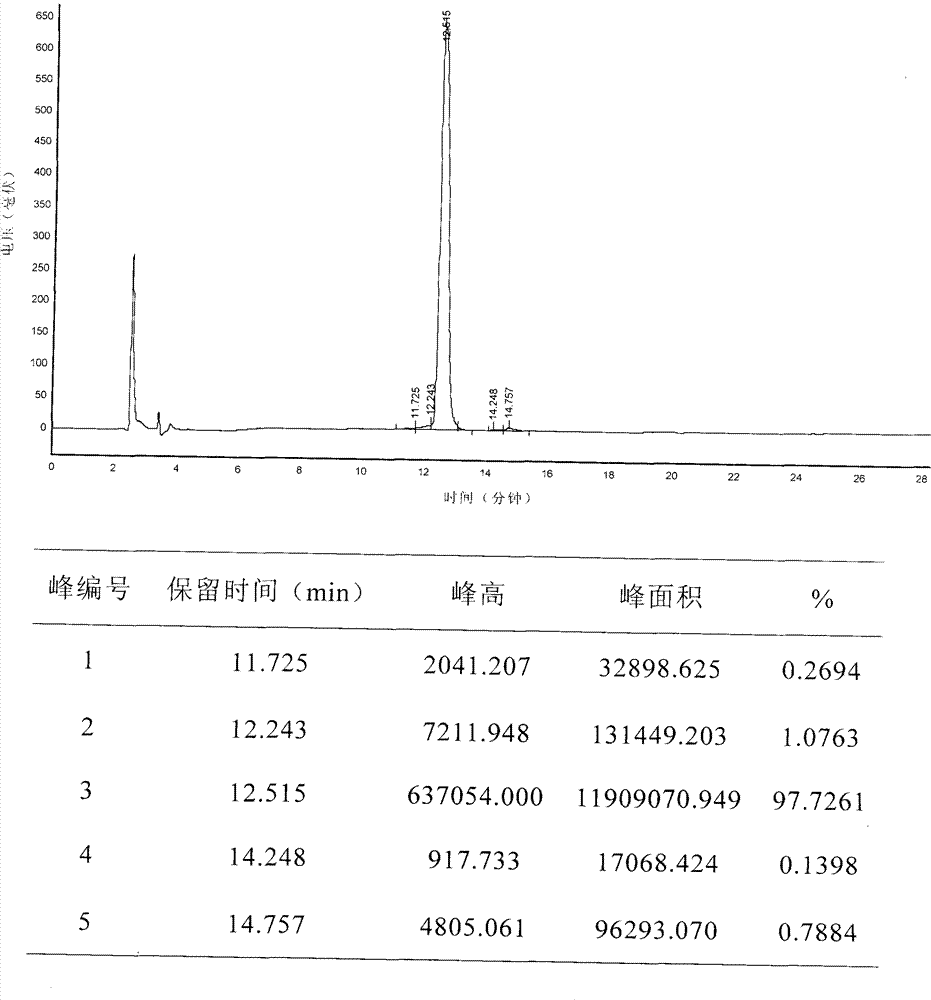
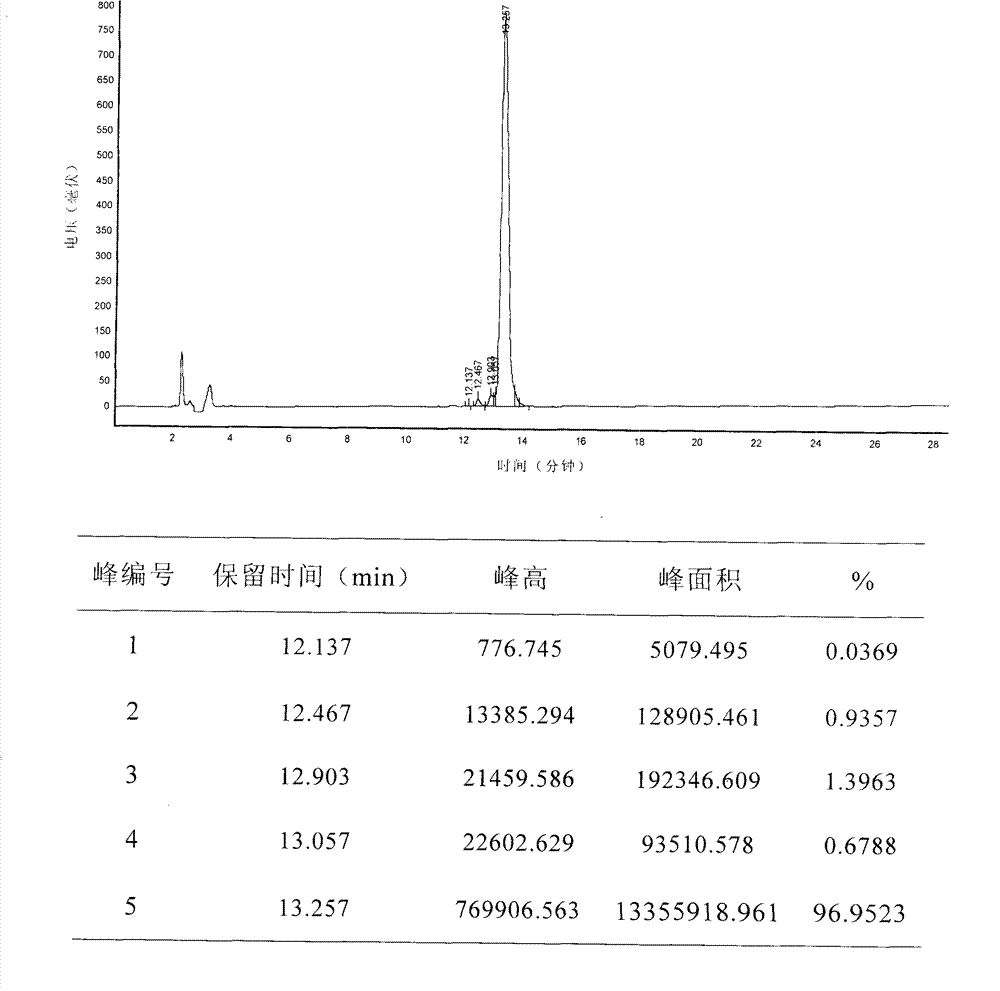
[0148] The purity and molecular weight detection of embodiment 3 polypeptide
[0149] The above synthesized polypeptide 1 and polypeptide 2 were determined by reverse phase chromatography (RP-HPLC) respectively, and the specific method is as follows:
[0150] 4.6×250mm5μm C18 analytical column (Kromasil);
[0151] Mobile phase A is trifluoroacetic acid (trifluoroacetic, TFA) added to 100% acetonitrile (acetonitrile, ACN), so that the concentration of TFA is 0.1% (v / v);
[0152] Mobile phase B is TFA added to 100% water, so that the concentration of TFA is 0.1% (v / v);
[0153] The flow rate is 1.0ml / min;
[0154] The detection wavelength is 220nm;
[0155] Elution gradient: The ratio of mobile phase A to the total volume of A and B is 15% (v / v). After injection, linear gradient elution (Gradient Elution) is used, and mobile phase A accounts for A within 25 minutes. The ratio of the total volume of the two items of B and B was changed from 15% (v / v) to 50% (v / v), and then eq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com