Crystal of doripenem intermediate and preparation method thereof
A technology for doripenem and intermediates, which is applied in the field of crystallization of pharmaceutical intermediates and their preparation, can solve the problems of high cost, unfavorable industrialization and the like, and achieve the effects of easy crystallization, good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] Preparation of Reference Example 1 Compound (I)
[0028]
[0029] According to the method described in Japanese Patent 5-294970, under nitrogen protection, add formula (III) compound (28.5g, 0.11mol), triphenylphosphine (33.2g, 0.127mol), formula (II) compound (25.9g, 0.132mol), add 200ml of dry ethyl acetate to dissolve and mix, cool in an ice bath to about 0°C, slowly add DIAD (diisopropyl azodicarboxylate) (26.7g, 0.132mol) dropwise, and stir at room temperature for about 12 After hours, the precipitated white solid (triphenoxyphosphine) was removed by filtration, and the solvent was removed under reduced pressure to obtain about 80.0 g of a yellow oil.
Embodiment 1~8
[0030] The preparation of the crystal of embodiment 1~8 compound (I)
[0031] The compound (I) crystals were prepared with soluble solvent and water, and the results are listed in Table 1.
[0032] The specific operation of each embodiment is as follows: 10 g of the yellow oil obtained in Reference Example 1 was dissolved in a soluble solvent, and after the solution was dissolved, water was continuously added dropwise thereto, and the solution was stirred at 10°C to 40°C for several hours .
[0033] Table 1
[0034] implement
example
Soluble
agent
ml
insoluble
solvent
ml
result
(g)
analysis results
hour
between
temperature
purity
1
5.0
water
5.0
+3.1
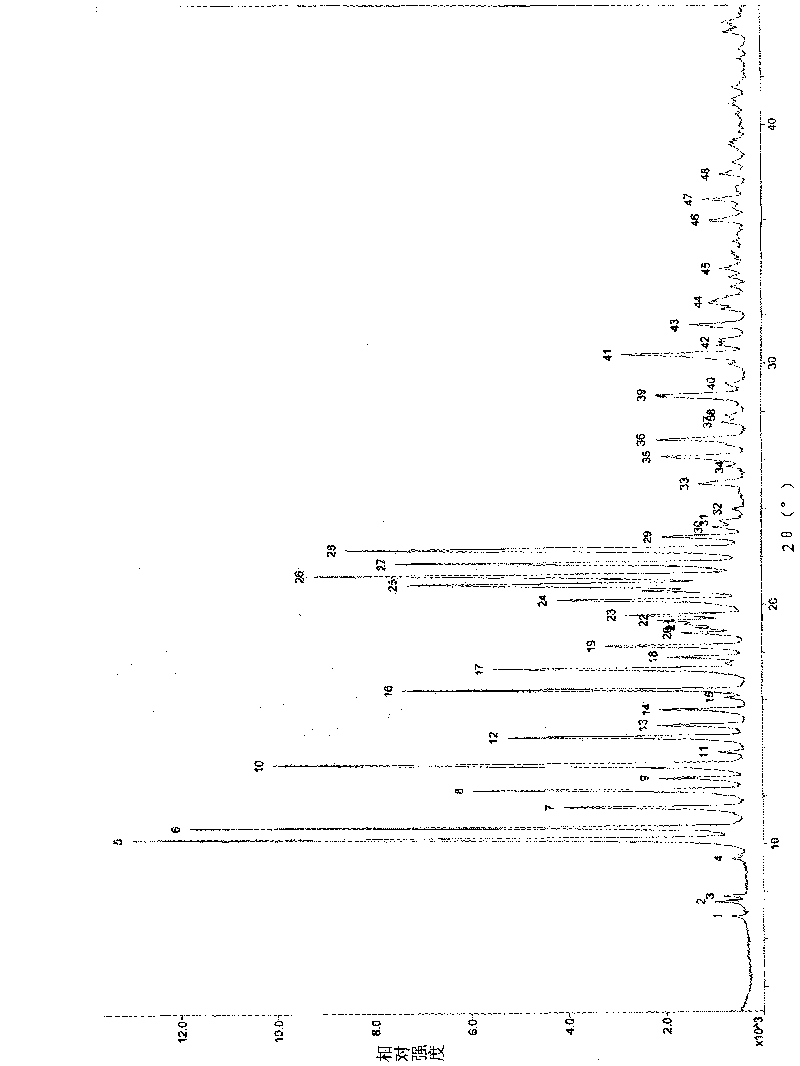
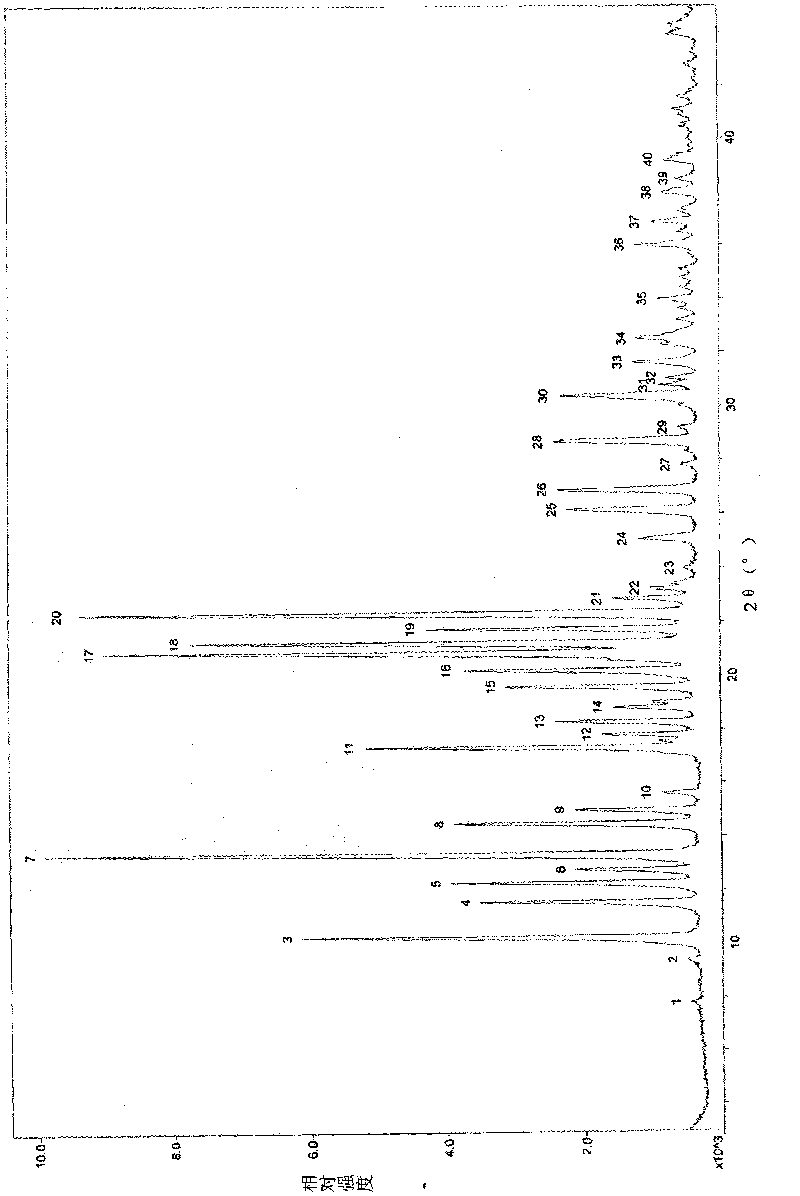
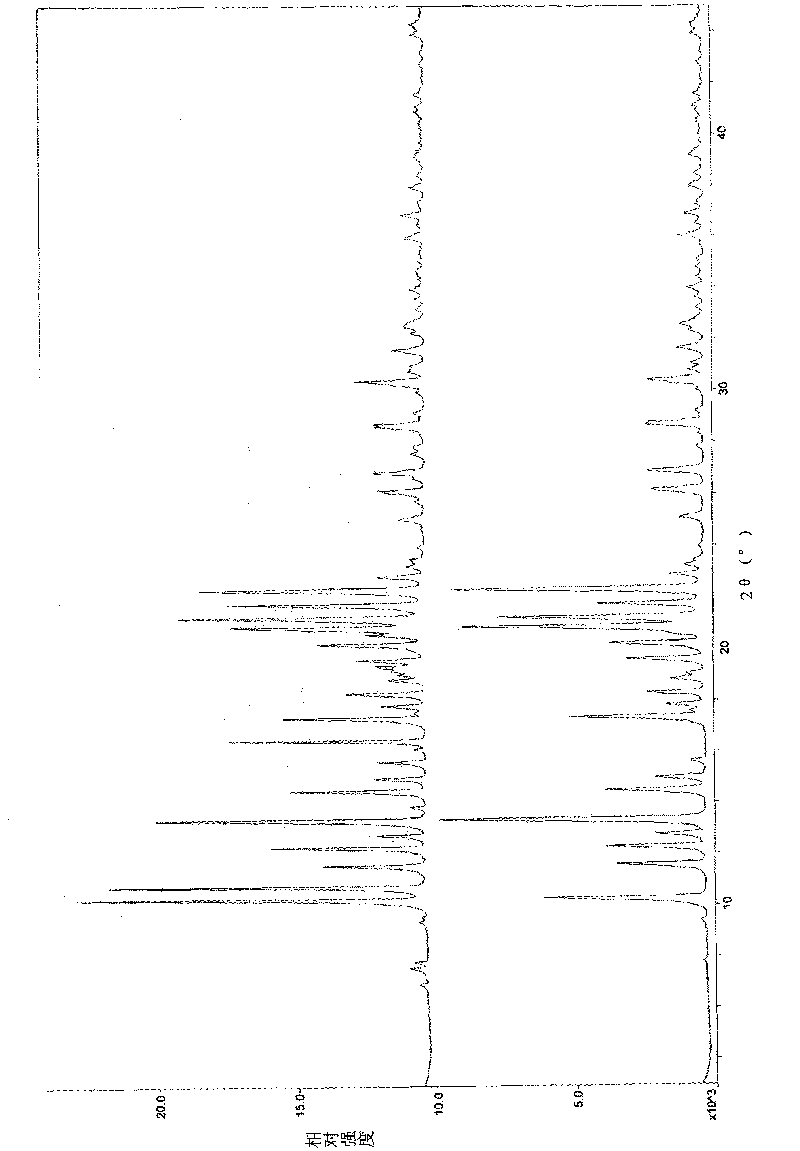
attached figure 1 and table 3
4h
20℃
96.0%
2
8.0
water
8.0
+3.6
attached figure 1...
Embodiment 9~14
[0038] The preparation of the crystal of embodiment 9~14 compound (I)
[0039] Compound (I) crystals were prepared with methanol, and the results are listed in Table 2.
[0040] The specific operations of each embodiment are as follows: 10 g of the yellow oil obtained in Reference Example 1 is dissolved in methanol, wherein the volume-to-mass ratio of methanol to the mixture is 0.5 to 2.0 ml / g, and the solution is heated at 10° C. to 30° C. Stir for several hours.
[0041] Table 2
[0042] implement
example
Soluble
agent
ml
result
(g)
Powder X-ray Diffraction Analysis Results
fruit
hour
between
temperature
purity
9
5.0
+2.5
attached figure 1 and table 3
4h
20℃
95.0%
10
Methanol
8.0
+2.8
attached figure 1 and table 3
6h
30℃
96.5%
11
Methanol
12
+1.5
Attached fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com