N-substituent thiazolone derivative and preparation method and applications thereof
A technology based on thiazolone and its derivatives, applied in the field of organic chemistry, can solve the problems of reduced or no harvest in agricultural production, unsatisfactory results, etc.
Inactive Publication Date: 2010-06-23
SHANDONG NORMAL UNIV
View PDF52 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
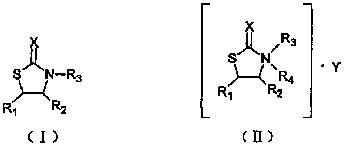
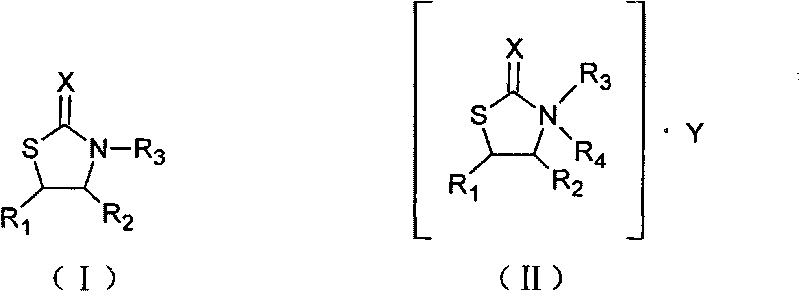
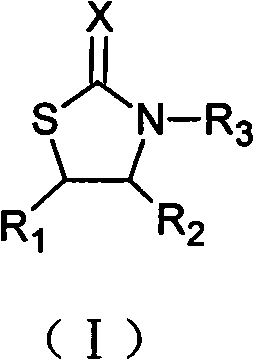
The invention relates to an N-substituent thiazolone derivative. The chemical structure of the thiazolone derivative is described as the following formula (I) or formula (II), wherein R1, R2, R3, R4, X and Y groups are detailed in the specification. The N-substituent thiazolone derivative synthetized by the invention has bactericidal activity on sclerotium sclerotiorum, blumeria graminis and the like, has antibacterial activity on staphylococcus aureus, colon bacillus, hay bacillus and the like, and has higher activities than the corresponding thiazolone compound.
Description
technical field The invention relates to an N-substituent thiazolone derivative, in particular to an N-substituent thiazolone derivative with bactericidal effect, and belongs to the technical field of organic chemistry. Background technique Agricultural fungicides refer to chemical agents that can directly kill the spores and mycelium of fungi or bacteria, or inhibit the growth and development of pathogens. The principle of sterilization is to give priority to prevention, supplemented by prevention and control, that is, active prevention and control should be carried out before or at the early stage of harm caused by germs. If the disease has been harmed or caused harm, and then prevent and control, even if the best fungicide is used, the effect will not be satisfactory. Sometimes it even reaches a level that is difficult to control, resulting in reduced or no harvest in agricultural production. The international fungicide market has grown rapidly, with an average annual g...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D277/16C07D277/14A01N43/78A01P3/00A01P1/00
Inventor 刘玉法刘秀明王锐刘军伟张丽伟
Owner SHANDONG NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com