Phycocyanin beta subunits fluorescent protein combined with phycoerythrobilin PEB and application thereof
A technology of phycocyanin and phycoerythrin, applied in application, algae/moss peptides, hybrid peptides, etc., can solve the problems of complicated technical procedures, high background, difficult quantitative determination by fluorescence immunology technology, etc., and achieve fluorescence efficiency. High, easy to purify, good sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
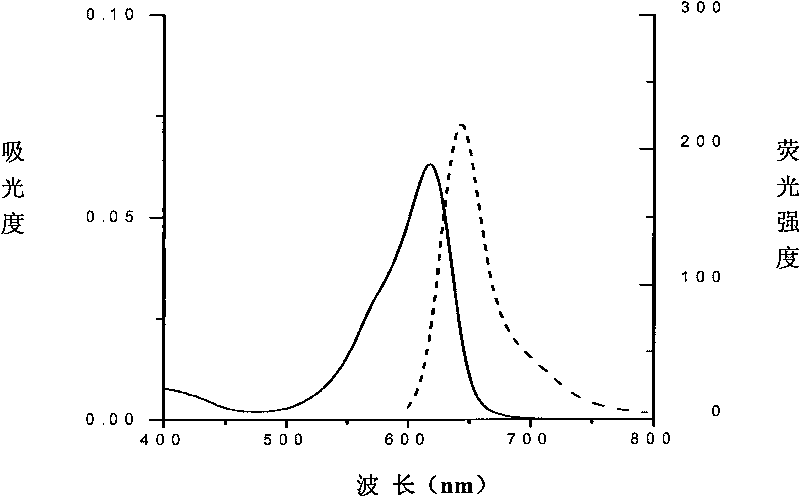
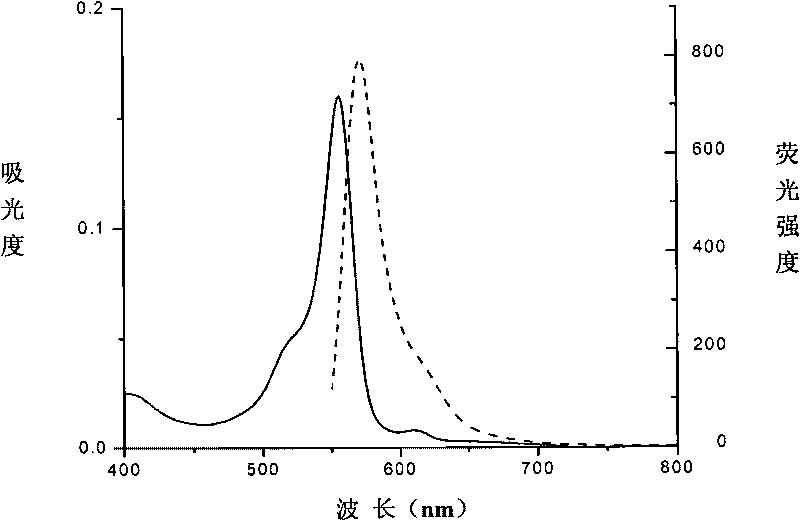
[0056] The amino acid sequence of the protein is shown in Sequence 1. The gene encoding the phycocyanin beta subunit is cloned into an expression plasmid, and the phycocyanin beta subunit is expressed, and its N-terminal has a His-tag mark, which is not only conducive to its purification , also helps to improve its solubility. Phycoerythrin is bound to the cysteine residue at position 130 (equivalent to position 82 of the original phycocyanin beta subunit) through a thioether bond. Its spectrum is as figure 2 As shown, the absorption peak is 556nm, and the fluorescence emission peak is 572nm.
Embodiment 2
[0058] The amino acid sequence of the protein is shown in Sequence 2. The gene encoding the phycocyanin beta subunit is cloned into an expression plasmid, and mutated by genetic engineering methods to obtain a phycocyanin beta subunit mutant with His at its N-terminus. -tag mark, which not only facilitates its purification, but also helps to improve its solubility. Phycoerythrin is bound to the cysteine residue at position 130 (equivalent to position 82 of the original phycocyanin beta subunit) through a thioether bond. Its spectrum is as image 3 As shown, the absorption peak is 556nm, and the fluorescence emission peak is 572nm.
Embodiment 3
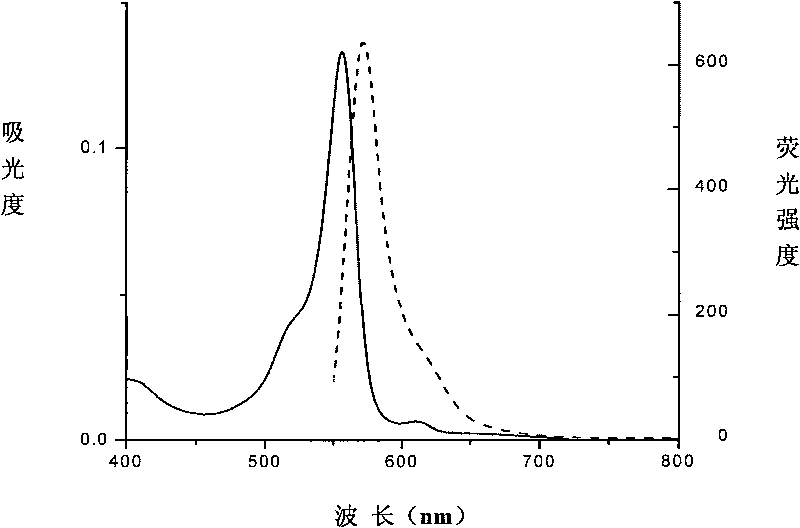
[0060] The amino acid sequence of the protein is shown in Sequence 3. The streptavidin coding gene and the phycocyanin beta subunit coding gene are spliced and cloned into the expression plasmid, and the fusion of streptavidin and phycocyanin beta subunit is expressed protein, directly achieve streptavidin labeling of phycocyanin beta subunit; and the N-terminal is marked with His-tag, which not only facilitates its purification, but also helps to improve its solubility. Phycoerythrin is bound to the cysteine residue at position 258 (equivalent to position 82 of the original phycocyanin beta subunit) through a thioether bond. Its spectrum is as Figure 4 As shown, the absorption peak is 556nm, and the fluorescence emission peak is 572nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com