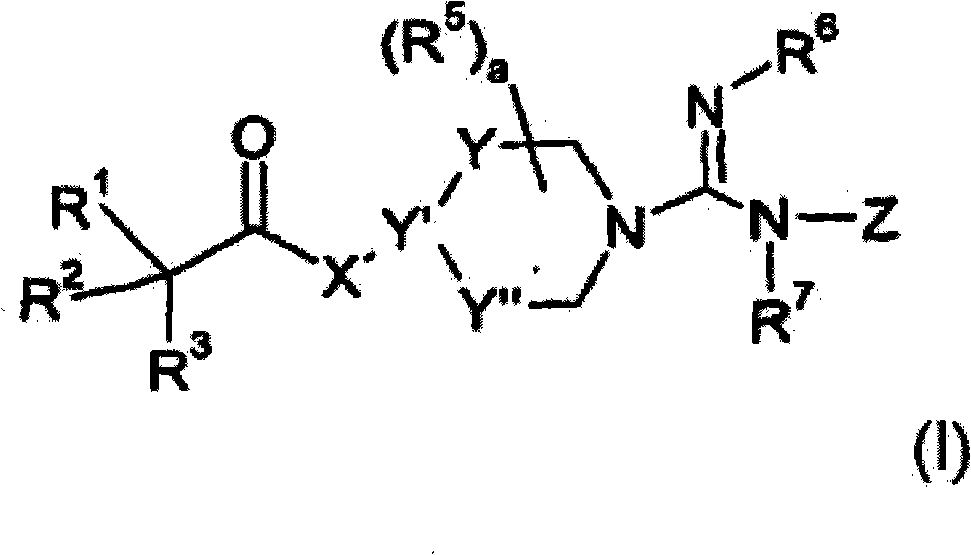
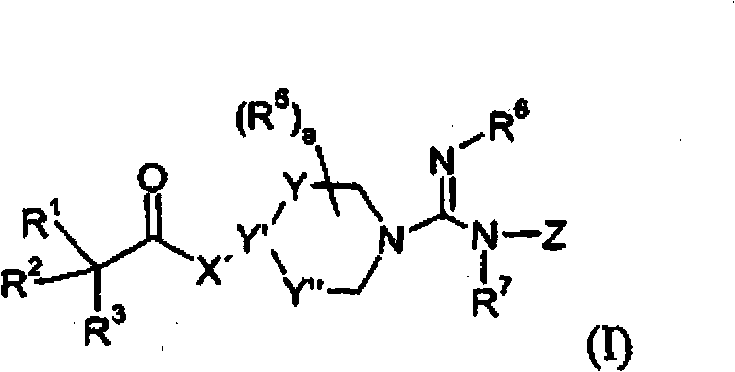
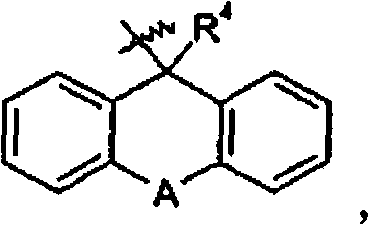
Guanidine-containing compounds useful as muscarinic receptor antagonists
A compound and alkyl technology, applied in the field of treatment of lung diseases, can solve the problems of cardiovascular side effects, dry mouth, insufficient treatment, etc., and achieve the effects of large therapeutic window, long receptor half-life, and improved binding affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0199] The following Preparations and Examples are provided to illustrate specific embodiments of the invention. However, unless expressly stated otherwise, these specific examples are not intended to limit the scope of the invention in any way.
[0200] Unless otherwise stated, the following abbreviations have the following meanings, and any other abbreviations not defined herein have their standard meanings:
[0201] AC adenylyl cyclase
[0202] BSA bovine serum albumin
[0203] cAMP 3′-5′ cyclic adenosine monophosphate
[0204] CHO Chinese Hamster Ovary
[0205] m 5 Cloned Chimpanzee M 5 receptor
[0206] DCM dichloromethane (i.e. methylene dichloride)
[0207] DIPEA N,N-Diisopropylethylamine
[0208] dPBS Dulbecco's phosphate buffered saline
[0209] DMF N,N-Dimethylformamide
[0210] EDCI N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide
[0211] EDTA ethylenediaminetetraacetic acid
[0212] EtOAc ethyl acetate
[0213] FBS fetal bovine serum
[0214] FLI...
example 1
[0241] 4-((R)-2-Cyclopentyl-2-hydroxy-2-phenylacetyl)-N-thiophen-2-ylmethylpiperazine-1-carboxamidine
[0242]
[0243] To a stirred solution of (R)-2-cyclopentyl-2-hydroxy-2-phenyl-1-piperazin-1-ylethanone (3.9 g 13.7 mmol) in DMF (200 mL) was added DIPEA (4.8 mL, 27.3 mmol), and then C-(bis-benzotriazol-1-yl)methyleneamine (3.6 g, 13.7 mmol) was added. It was stirred at room temperature for 30 minutes, then C-thiophen-2-yl-methylamine (2.8 mL, 27.3 mmol) was added. The mixture was heated at 60°C for about 14 hours. The reaction was cooled to room temperature, and the solvent was removed by reduced pressure. The crude material was purified by reverse phase-HPLC to obtain the title compound (0.7 g, 1.3 mmol) as TFA salt. MS m / z: [M+H] + The computed value is C 23 h 30 N 4 o 2 S, 427.21; experimental value 427.2.
[0244] alternative synthesis
[0245] Add DIPEA (7.3 mL, 41.6 mmol) to (R)-2-cyclopentyl-2-hydroxy-2-phenyl-1-piperazin-1-ylethanone dissolved in etha...
example 2
[0247] 4-((R)-2-cyclopentyl-2-hydroxy-2-phenylacetyl)-N-(4-hydroxybenzyl)piperazine-1-carboxamidine
[0248]
[0249] (R)-2-Cyclopentyl-2-hydroxy-2-phenyl-1-piperazin-1-ylethanone (5.00 g 17.3 mmol; prepared as described in Preparation 1) in DMF (200 mL ) was added DIPEA (10.6 mL, 60.7 mmol) followed by C-(bis-benzotriazol-1-yl)-methyleneamine (5.48 g, 20.8 mmol). It was stirred at room temperature for 30 minutes, then 4-hydroxybenzylamine (12.0 g mL, 97 mmol) was added. The mixture was heated at 60°C for about 14 hours. The reaction was cooled to room temperature, and the solvent was removed by reduced pressure. The crude material was purified by reverse phase-HPLC to obtain the title compound (1.7 g, 3.1 mmol) as TFA salt. MS m / z: [M+H] + The computed value is C 25 h 32 N 4 o 3 , 437.25; the experimental value is 437.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com