Method for preparing stable docetaxel submicron emulsion injection and freeze-dried emulsion
A technology for docetaxel and milk injections, which is applied in the direction of freeze-dried delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of precipitation, limited solubility, and increased particle size, etc. problem, to achieve the effect of increasing stability, good technical effect and improving stability
Active Publication Date: 2010-08-25
HAINAN BIKAI PHARM CO LTD
View PDF1 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Tween-80 is hemolytic, and intravenous injection will aggravate the allergic reaction of the patient, causing the patient to have dyspnea, angioedema, hypotension, rubella and other allergic reactions, as well as severe adverse reactions such as shock, and even death
Therefore, before and during the clinical application of docetaxel injection, a large amount of hormone drugs should be taken orally or intramuscularly for desensitization intervention. In this way, a considerable proportion of patients will still have allergic reactions, causing great pain to the patients
At present, there are many literatures and patent applications related to docetaxel microemulsions and lyophilized emulsions, and the oil used in some patent application prescriptions has limited dissolving ability relative to docetaxel, so that the formulation specifications cannot meet the clinical requirements. The minimum dosage, that is, the drug loading of the preparation is small; in terms of stability, such as the patent application of Chinese Patent Publication No. CN1709236A, the emulsion is finally sterilized by circulating steam at 110-120°C. Such a sterilization process will lead to more main drugs The content of cetaxel dropped sharply, the emulsion droplets aggregated, the particle size increased, and even the phenomenon of emulsion stratification and precipitation occurred, and the stability of the preparation was poor
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Login to View More
Abstract
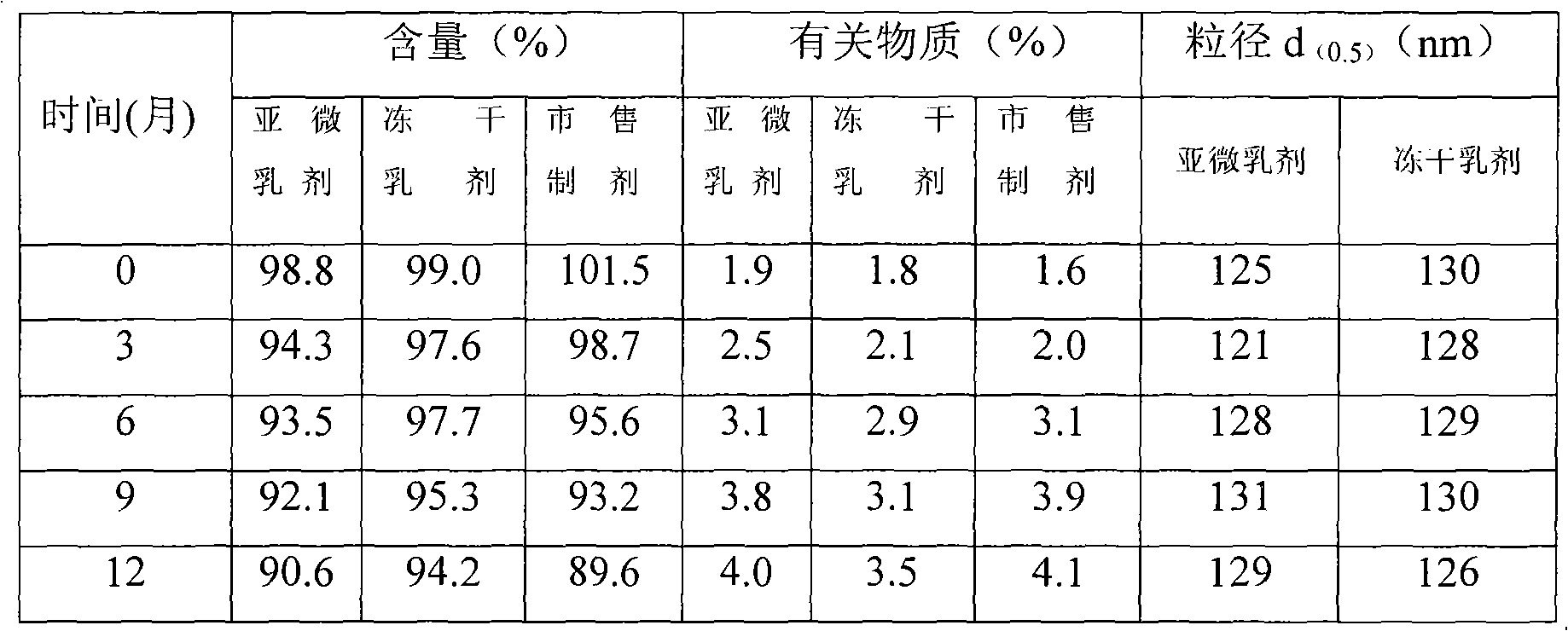
The invention discloses a method for preparing stable and high drug-loading rate intravenous docetaxel submicron emulsion injection and freeze-dried emulsion. A main active ingredient of the submicron emulsion is docetaxel, and the auxiliary materials are oil for injection, an emulsifying agent, a stabilizing agent, an isoosmotic adjusting agent, lyoprotectants and water for injection. The docetaxel content is 1 to 10mg / ml. The emulsion has small grain size, and the grain size range after the submicron emulsion injection and the freeze-dried emulsion are dissolved compositely is 100 to 200nm. By selecting proper oil phase and emulsifying agent to optimize the preparation process, the prepared docetaxel submicron emulsion injection and freeze-dried emulsion have the characteristics of high drug-loading rate, high stability, easy industrial production, strong clinical applicability and the like.
Description
Technical field: The invention belongs to the technical field of pharmaceutical preparations, and relates to a preparation method of docetaxel microemulsion injection and freeze-dried emulsion. Background technique: Docetaxel, also known as docetaxel, is a taxane compound with a chemical name: [2aR-(2aα, 4β, 4aβ, 6β, 9α, (aR * , βS * ), 11α, 12α, 12aα, 12bα)]-β-[[(1,1-dimethylethoxy)carbonyl]amino]-α-carbonylphenylpropanoic acid [12b-acetoxy-12-benzoyl Oxy-2a, 3, 4, 4a, 5, 6, 9, 10, 11, 12, 12a, 12b-dodecahydro-4, 6, 11-trihydroxy-4a, 8, 13, 13-tetramethyl -5-oxo-7,11-methylene-1H-cyclodecepenta[3,4]benzo[1,2-b]oxetan-9-yl] ester; its molecular formula is: C 43 h 53 NO 14 ;Molecular weight: 807.89. It is a product obtained by semi-synthesizing inactive compounds extracted from the needles of European berry yew. In 1994, it was successfully developed by the French Ronapque Lean Company, and it is used as a chemotherapy drug for breast cancer and non-small cell lung ca...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/107A61K9/19A61K31/337A61K47/14A61K47/34A61K47/44A61P35/00A61K47/10
Inventor 陈容史朝晖冯仲异
Owner HAINAN BIKAI PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com