Carbamido peptide aminopeptidase N inhibitor and application thereof
A urea group, amino acid technology, applied in the field of chemistry, can solve the problems of high cost of total synthesis, limited sources, pain and so on
Inactive Publication Date: 2010-09-01
SHANDONG UNIV
View PDF2 Cites 13 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
5) Aminopeptidase N participates in the degradation of endogenous analgesic substances endorphins and enkephalins, thereby causing excessive release of substance P, leading to pain
In addition, most of the inhibitors of aminopeptidase N in clinical or preclinical studies are natural products. For example, Ubenimex (Ubenimex), as a dipeptide-like structure containing β-amino acids, is currently used as an immune enhancer. For the treatment of leukemia, it is isolated from the culture fluid of Streptomyces olivorecticuli, and the total synthesis is expensive, so the source is limited
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
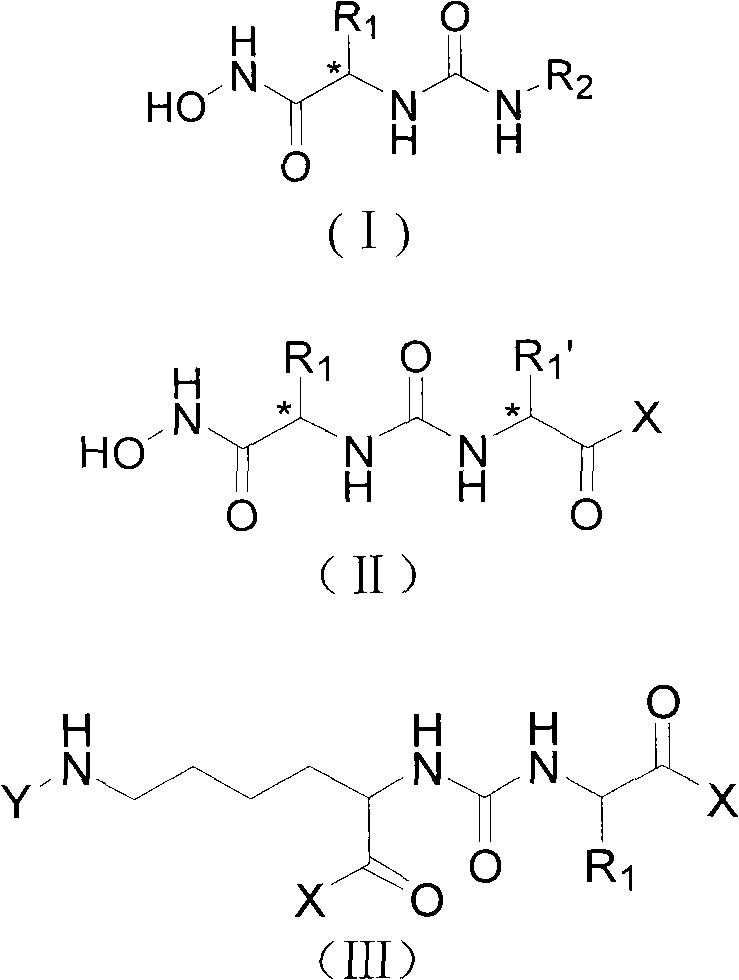
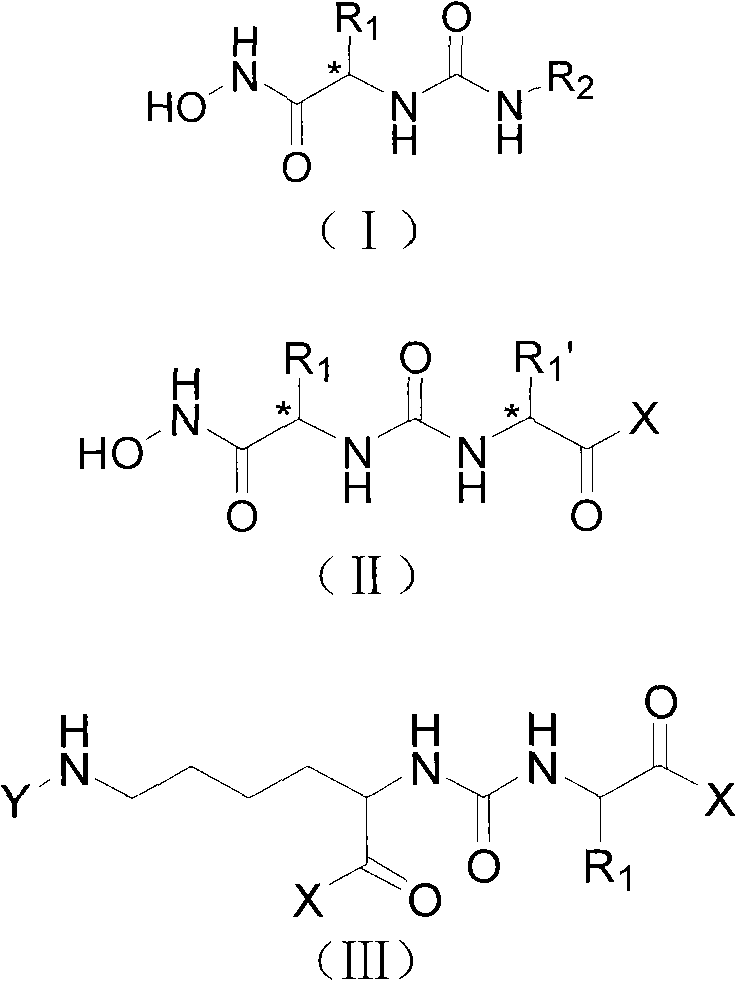
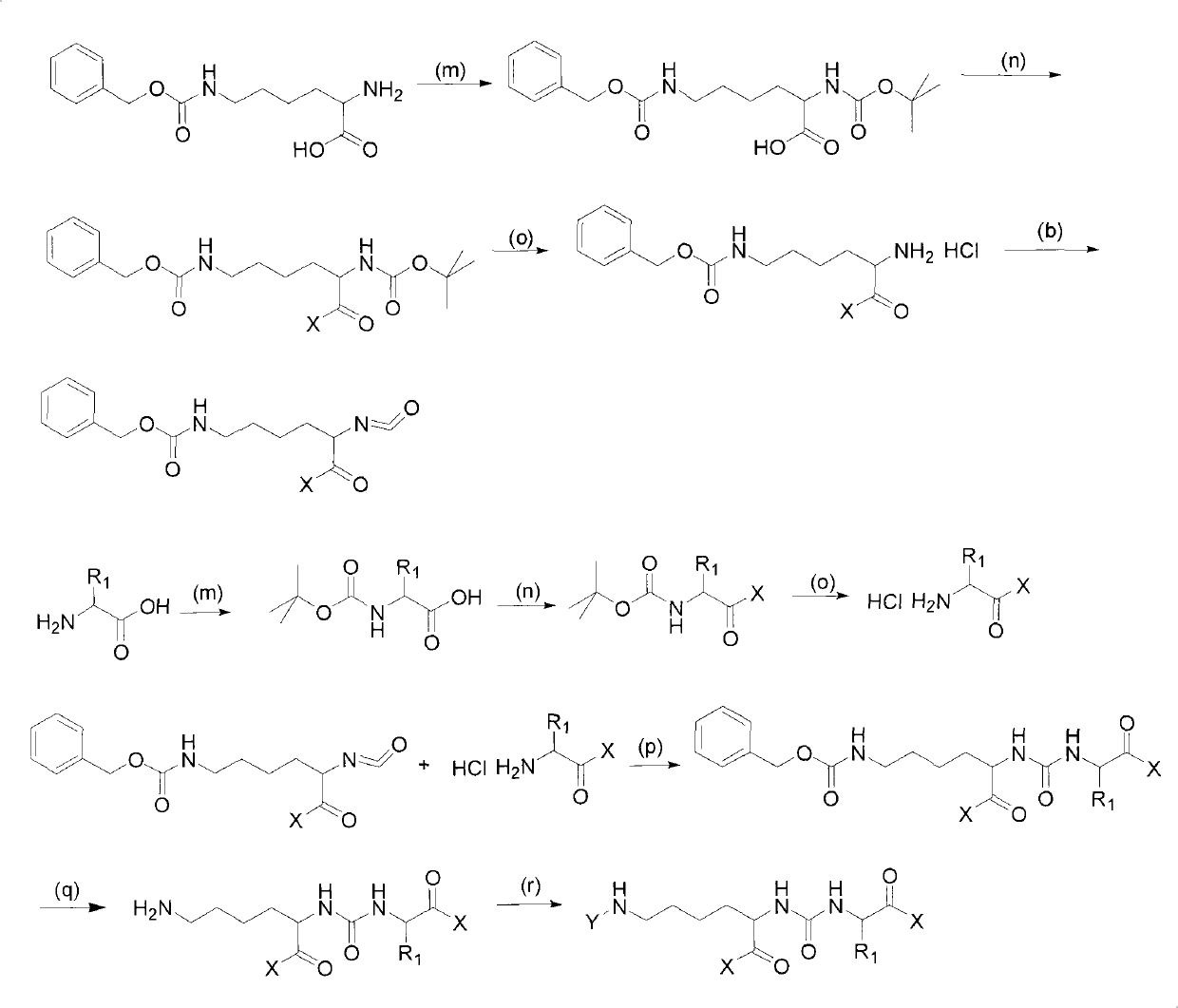
The invention provides a carbamido peptide aminopeptidase N inhibitor and application thereof. The invention provides the superactive peptide aminopeptidase N inhibitor, thereby being capable of curing the disease that the activity or the express of the aminopeptidase N is abnormal. Specifically, the invention relates to a peptide compound with the structures of general formulas (I), (II) or (III), and further relates to various optical isomers thereof, a pharmaceutically acceptable salt, a solvate and a prodrug. The invention further relates to a drug composite of the peptide compound which comprises the structures of the general formulas (I), (II) or (III) and use therefore for preparing the drug.
Description
technical field The invention relates to a ureido-peptide aminopeptidase N inhibitor, a preparation method and application thereof, and belongs to the field of chemical technology. Background technique Aminopeptidase N (APN, CD13) is a type II membrane-bound glycoprotein with a molecular weight of about 150Kd, belonging to the Gluzincins subfamily of the zinc ion-dependent metalloprotease and aminopeptidase M1 family, and exists in the form of homodimers In the cell membrane, it participates in the degradation of the N-terminal amino acid of the substrate. Aminopeptidase N is widely distributed in GM-SCF and myeloid cells at various stages of development; in non-blood cells, stromal cells distributed in hematopoietic organs, tumor vascular endothelial cells, various epithelial cells including renal proximal tubule epithelial cells, bile duct epithelial cells And small intestinal brush border epithelial cells, etc.; also distributed on the surface of fibroblasts, mesenchymal...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C323/60C07C323/59C07C275/24C07C275/16C07C319/20C07C273/18A61K31/17A61K31/223A61K31/27A61K31/198A61P35/00A61P35/02A61P1/04A61P1/02A61P17/00A61P25/28A61P37/02
Inventor 徐文方宿莉方浩
Owner SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com