Phenobarbital derivatives useful in immunoassay
A phenobarbital, medium phenobarbital technology, applied in the field of phenobarbital derivatives that can be used in immunoassays, can solve problems such as not pointing out barbiturate cross-reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
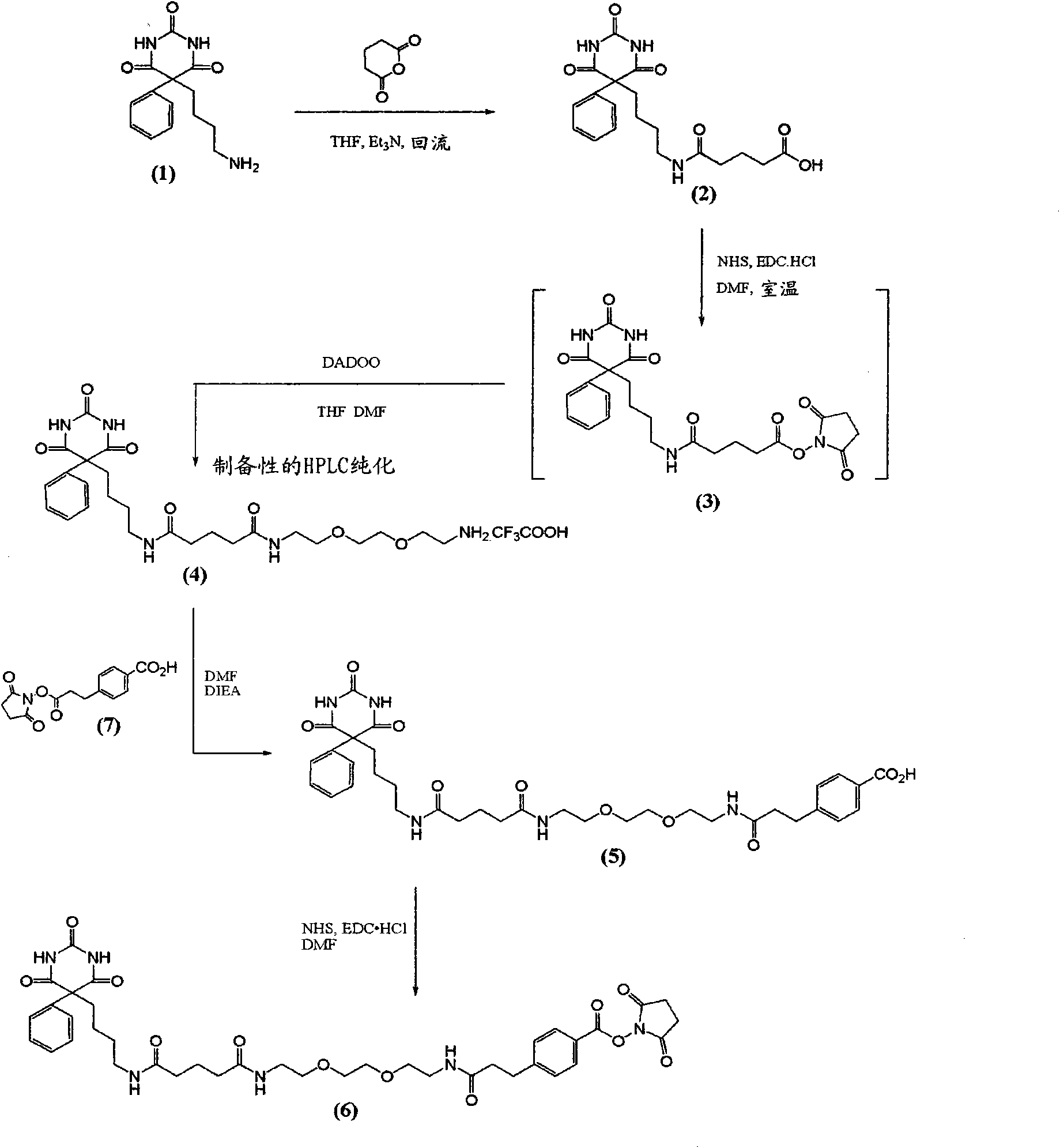
[0085] The synthesis of embodiment 1. compound (1)
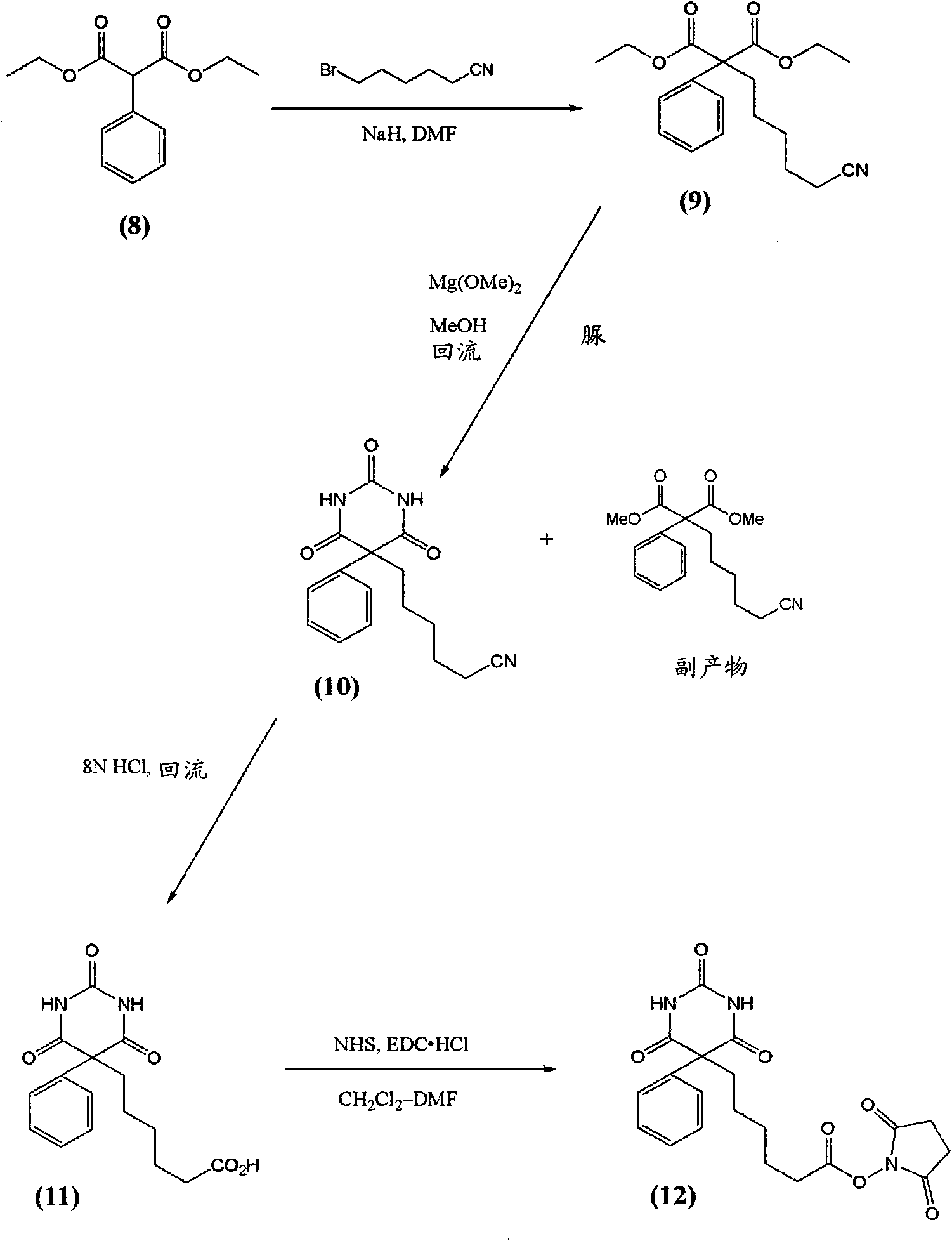
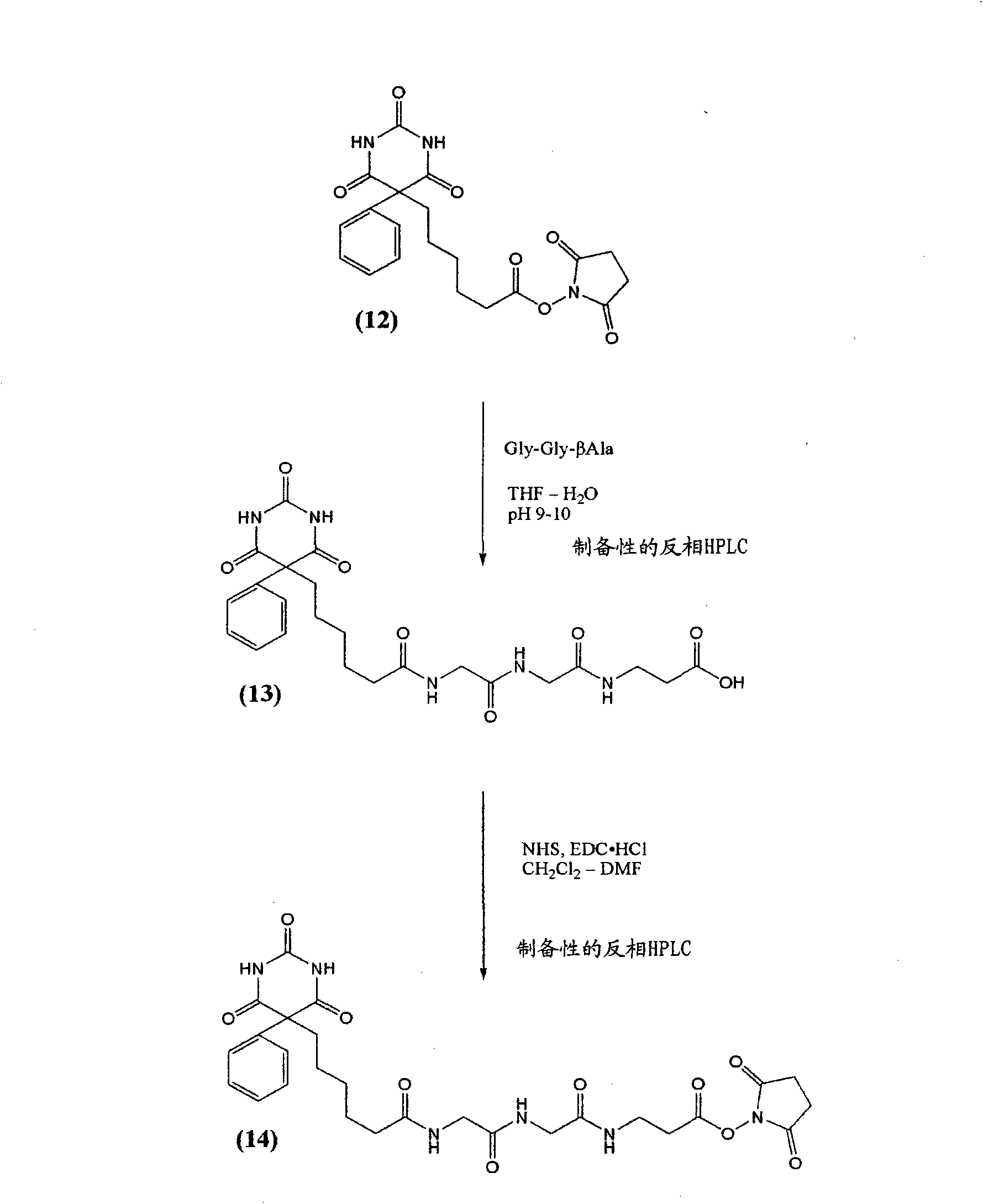
[0086] At room temperature, to 101 mg (0.259 mmol) of compound (1) [a] Krausz, L.M.; Hitz, J.B.; Buckler, R.T. and Burd, J.F. Therapeutic Drug Monitoring, 1980, 2, 261-272; b] Castro, A. ; Chung, A. and Monji, N. Research Communications in Chemical Pathology and Pharmacology, 1980, 28, 309-317.] In a stirred solution in 15 mL of dry THF containing 76 μL (0.55 mmol; 2.1 equivalents) of triethylamine 47 mg (0.41 mmol; 1.6 equiv) of glutaric anhydride were added. After stirring briefly at room temperature, the reaction was boiled at reflux (90° C. oil bath) under argon. After 1 hour, analysis by RP-HPLC indicated that the reaction was complete with only one major product peak. The reaction was evaporated to dryness under reduced pressure (rotary evaporator). The residue was redissolved in about 2.5 mL of 1:1 MeCN / water, filtered (0.45 μ) and purified using preparative RP-HPLC [column I] using 5% (0 min) → 100% (20 min) → 100...
Embodiment 2
[0087] Embodiment 2. the synthesis of compound (3)
[0088] 16.1 mg (0.140 mmol) of N-hydroxysuccinimide (NHS) and 38.1 mg (0.199 mmol) of 3-ethyl-1-dimethylaminopropylcarbodiimide hydrochloride were mixed under argon (EDC.HCl) was added to a stirred solution of 54.1 mg (0.139 mmol) of compound (2) in 4 mL of dry DMF and the solution was stirred at room temperature under argon for about 3 h. LC / MS indicated partial NHS ester formation. A further 32.3 mg (0.281 mmol; total addition = 0.421 mmol, 3 eq) of NHS and 55.3 mg (0.288 mmol; total addition = 0.487 mmol, 3.5 eq) of EDC.HCl were added and the reaction was heated at room temperature under argon Stir overnight. LC / MS indicated essentially complete conversion to the desired NHS ester (3). LC / MS: t R ~10.0 min [5% (0 min) → 88% (17.5 min) of 0.1% TFA / MeCN in 0.1% TFA / water]; found M+H 487.1. The entire product mixture / solution was used as such in the next step [see Example 3] without further purification.
Embodiment 3
[0089] Embodiment 3. the synthesis of compound (4)
[0090] The entire reaction / product mixture (~4 mL) containing (3) (from Example 2; transferred via syringe to a small addition funnel) was added dropwise to 81.2 μL (~0.554 mmol; ~0.554 mmol; ~ 4 equivalents) of 2,2'-(ethylenedioxy)-diethylamine [Fluka 03739] (DADOO) in 4 mL of dry THF and 2 mL of dry DMF [using a pipette] while continuing Stir effectively. The flask and addition funnel from Example 2 were washed with 4 mL of dry THF and the washing was added dropwise to the reaction over ~15 min. Analysis of the reactants after ~0.5 h showed the disappearance of the NHS ester (3) and formation of the desired product (4) (t R ~8.25min), which contains a small amount of dimer. The reaction was evaporated under reduced pressure to remove THF, then under high vacuum (high vacuum rotary evaporator) to remove DMF. The residue was redissolved in 1:1 MeCN / water, filtered (0.45 μ) and purified using preparative RP-HPLC with 5% (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com