Method for preparing micro fine precipitated barium sulfate and co-producing hydrochloric acid by using barium slag
A technology for precipitating barium sulfate and slag, applied in the direction of calcium/strontium/barium sulfate, chlorine/hydrogen chloride purification, chlorine/hydrogen chloride, etc., can solve the problems of high cost, huge production equipment, complicated production process, etc. The effect of low equipment cost and high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
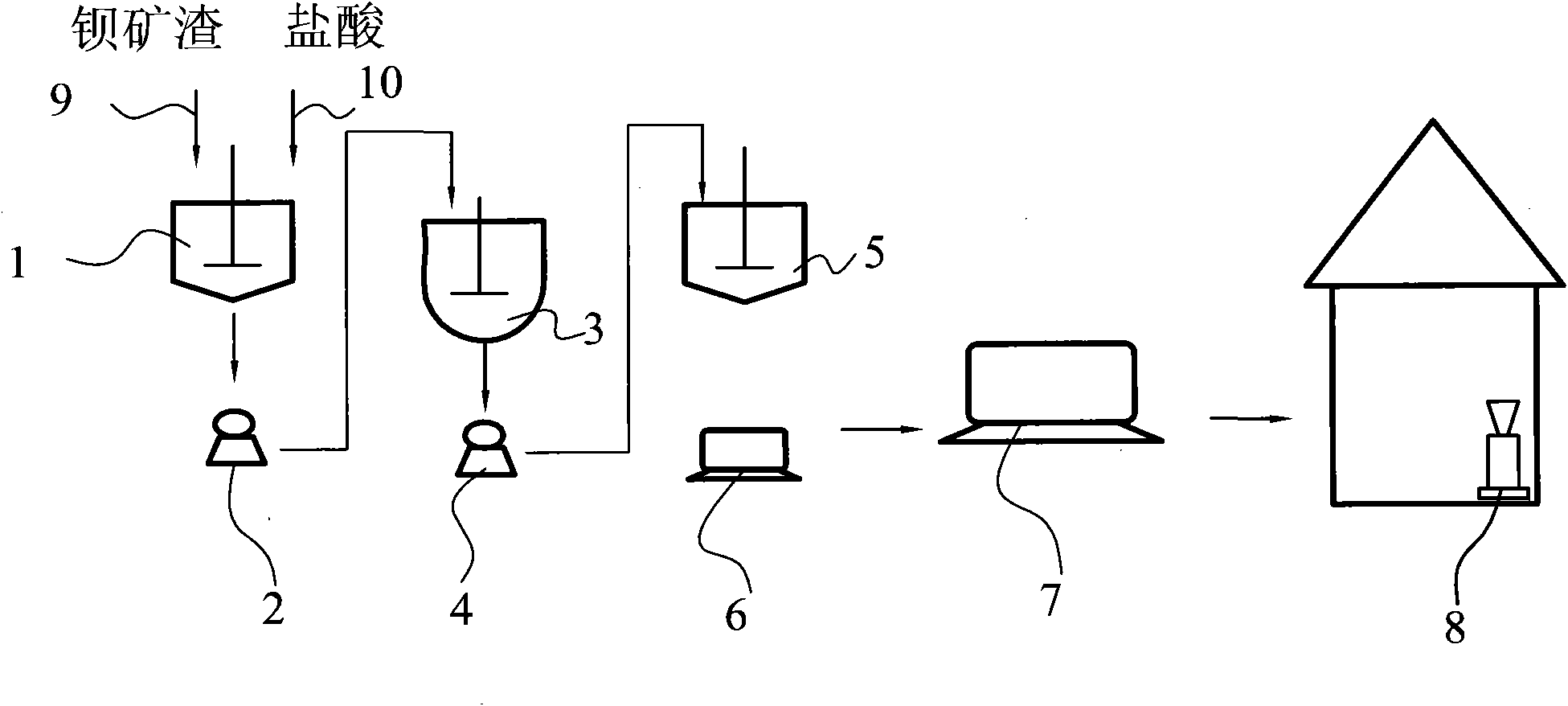
[0032] A method for preparing finely precipitated barium sulfate and co-producing hydrochloric acid with barium slag, wherein the barium slag is acidified, recovered and synthesized to produce finely precipitated barium sulfate, and hydrochloric acid product is co-produced at the same time;
[0033] The acidizing process is as follows: first inject 800 kilograms of barium slag and hydrochloric acid into acid leaching tank 1 through barium slag feeding line 9 and hydrochloric acid feeding line 10 respectively, and barium slag and hydrochloric acid are calculated as pure substances in the acid leaching tank The mass ratio is 1: 0.13, after reacting for 9 hours, use the first suction filtration pump 2 suction filtration again, the filtrate after the suction filtration is 341.92 kilograms of chlorides based on barium;
[0034] The recovery process is as follows: first the filter residue after suction filtration in the acidification process is moved into the recovery device, and the...
Embodiment 2
[0037] A method for preparing finely precipitated barium sulfate and co-producing hydrochloric acid with barium slag, wherein the barium slag is acidified, recovered and synthesized to produce finely precipitated barium sulfate, and hydrochloric acid product is co-produced at the same time;
[0038] The acidizing process is as follows: first inject 800 kilograms of barium slag and hydrochloric acid into acid leaching tank 1 through barium slag feeding line 9 and hydrochloric acid feeding line 10 respectively, and barium slag and hydrochloric acid are calculated as pure substances in the acid leaching tank The mass ratio is 1: 0.35, while stirring, the rotating speed of the stirrer is 280 rev / min, after reacting for 7 hours, then use the first suction filtration pump 2 for suction filtration, the filtrate after suction filtration is the chloride based on barium 797.8 kg;
[0039] The recovery process is as follows: the filter residue after suction filtration in the acidificatio...
Embodiment 3
[0042] A method for preparing finely precipitated barium sulfate and co-producing hydrochloric acid with barium slag, wherein the barium slag is acidified, recovered and synthesized to produce finely precipitated barium sulfate, and hydrochloric acid product is co-produced at the same time;
[0043] The acidizing process is as follows: first inject 800 kilograms of barium slag and hydrochloric acid into acid leaching tank 1 through barium slag feeding line 9 and hydrochloric acid feeding line 10 respectively, and barium slag and hydrochloric acid are calculated as pure substances in the acid leaching tank The mass ratio is 1: 0.53, after reacting for 9 hours, use the first suction filtration pump 2 suction filtration again, the filtrate after the suction filtration is 844.68 kilograms of chlorides based on barium;
[0044] The recovery process is as follows: the filter residue after suction filtration in the acidification process is first moved into the recovery device, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com