Thioxanthone photoinitiator containing cyclic acetal and preparation method thereof
A thioxanthone photo, cyclic acetal technology, applied in organic chemistry and other directions, can solve the problems of toxic carcinogenicity, restricted application, yellowing of cured films, etc., and achieves low preparation cost, reduction of yellowing and toxicity, Simple to use effects
Inactive Publication Date: 2010-10-20
HANGZHOU INST OF ADVANCED MATERIAL BEIJING UNIV OF CHEM TECH
View PDF3 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, amine compounds are toxic and carcinogenic, and the cured film caused by them is prone to yellowing
Restricts its application in food and drug packaging
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
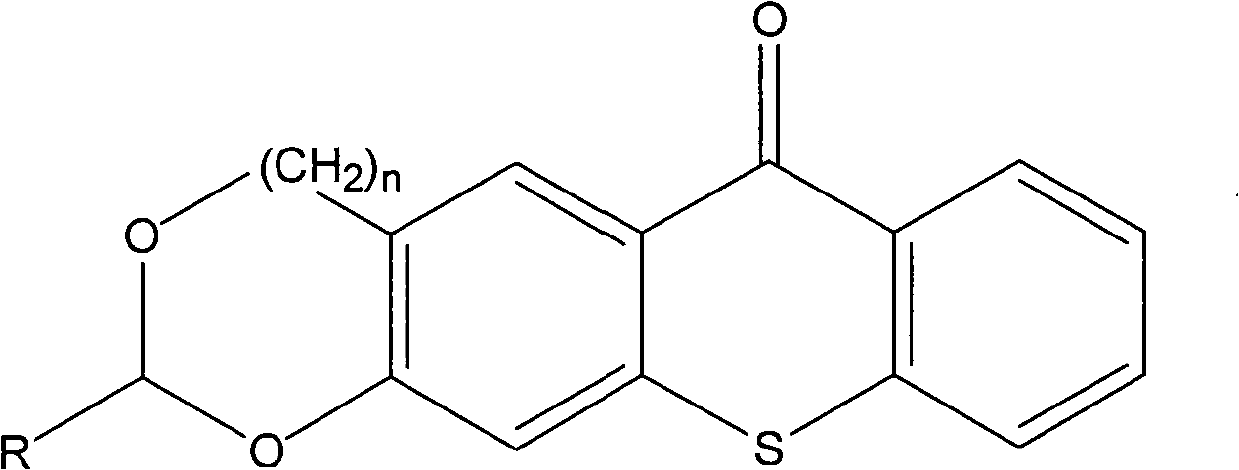
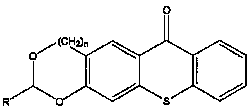
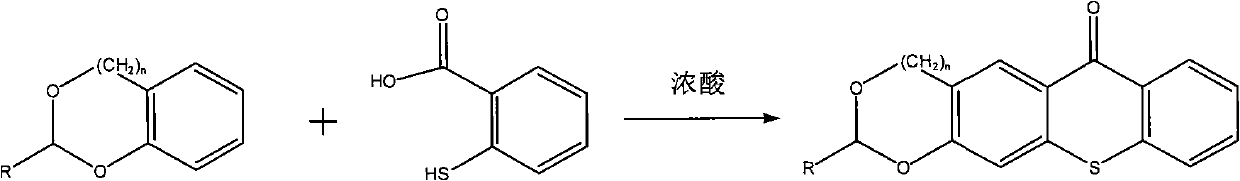
The invention discloses a thioxanthone photoinitiator containing cyclic acetal shown as below and a preparation method thereof. The 1,3-benzodioxolane or 1,3-benzodioxane and derivative thereof and 2-thiosalicylic acid are used as raw materials and concentrated sulphuric acid is used as solvent to react at 0-70 DEG C for 1-24 hours and prepare the thioxanthone containing cyclic acetal. As cyclic acetal group is introduced in thioxanthone and cyclic acetal can be used as the co-initiator of thioxanthone, the compound of the invention can be directly used as photoinitiator to replace the thioxanthone / amine initiation system and reduce the yellowing and toxicity caused by amine co-initiator and have wide application prospect in the photocuring industrial field.
Description
Technical field The invention belongs to a photoinitiator and a preparation method thereof, in particular to a thioxanthone photoinitiator containing a ring acetal and a preparation method thereof. Background technique The photopolymerization reaction of multifunctional monomers such as bis(meth)acrylate can quickly form a highly cross-linked polymer network structure. This feature is useful for curing speed, mechanical strength and stability of cured film in industrial coatings, dental restoration materials, etc. Areas with high sexual requirements are particularly important. Photoinitiator is a key component of the light curing system. It is related to whether the oligomer and diluent of the formula system can quickly change from liquid to solid when light is irradiated. Its basic function characteristics are: the initiator molecule has a certain light absorption ability in the ultraviolet light region (250-400nm) or visible light region (400-800nm). After directly or indire...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C08F2/48C07D495/04
Inventor 马贵平聂俊吴浩
Owner HANGZHOU INST OF ADVANCED MATERIAL BEIJING UNIV OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com