Laminins, derivatives, and compositions including same and method for their therapeutic use
A laminin, therapeutic technology, applied in the field of laminin, derivatives and compositions containing them and their therapeutic applications, can solve the problems of inability to provide therapy, limiting protein bioavailability, large size, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] Materials and Methods
[0159] animal
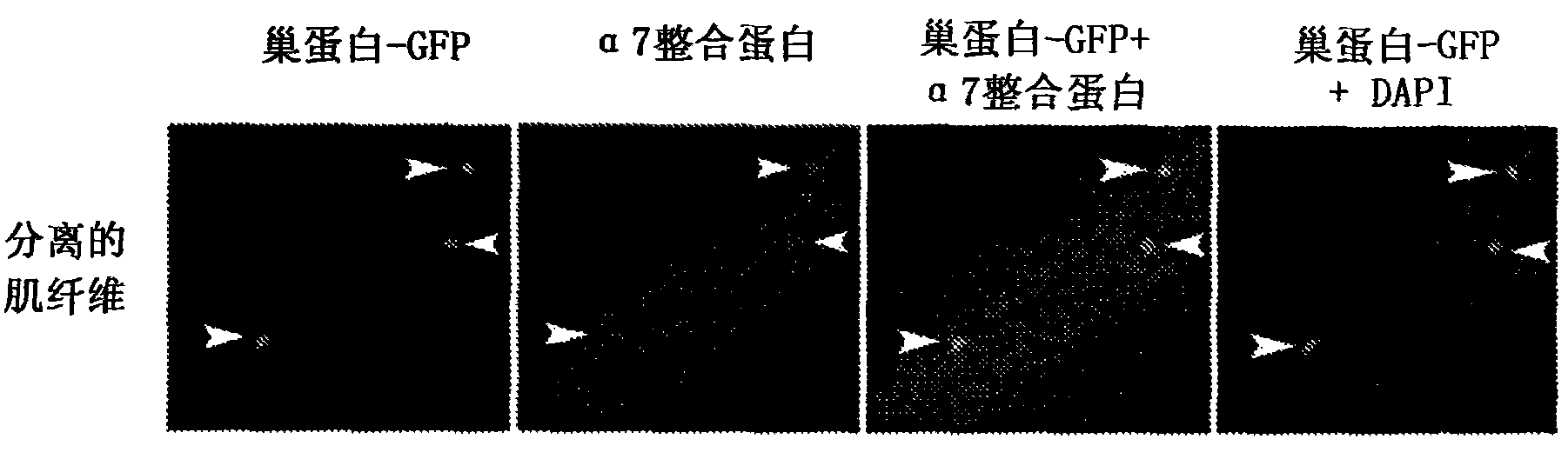
[0160] Wild-type (C57BL / 6), α7 integrin-deficient (C57BL / 6 background) and nestin-GFP mice (C57BL / 6 background) used in these studies, according to the University of Nevada (University of Nevada, Reno), Euthanasia was performed according to a protocol approved by the Institutional Animal Care and Use Committees of the University of Washington, Seattle.
[0161] Histology
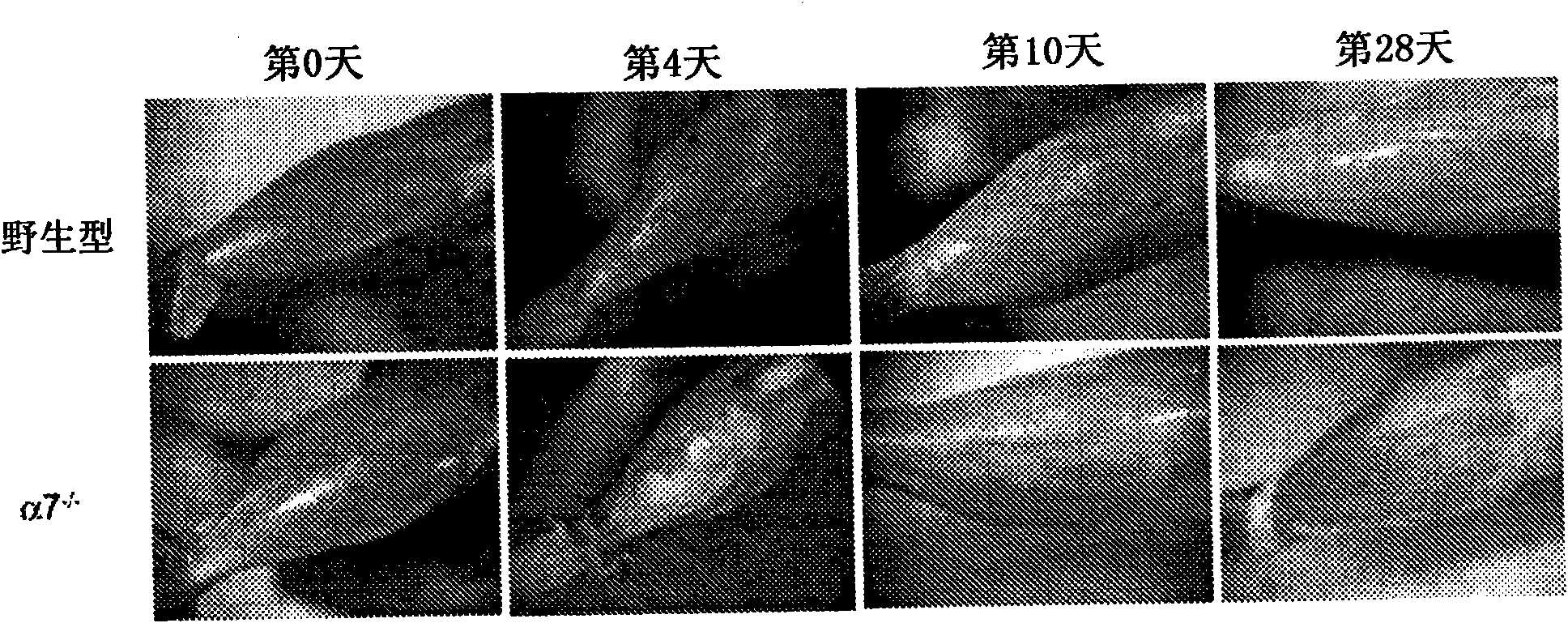
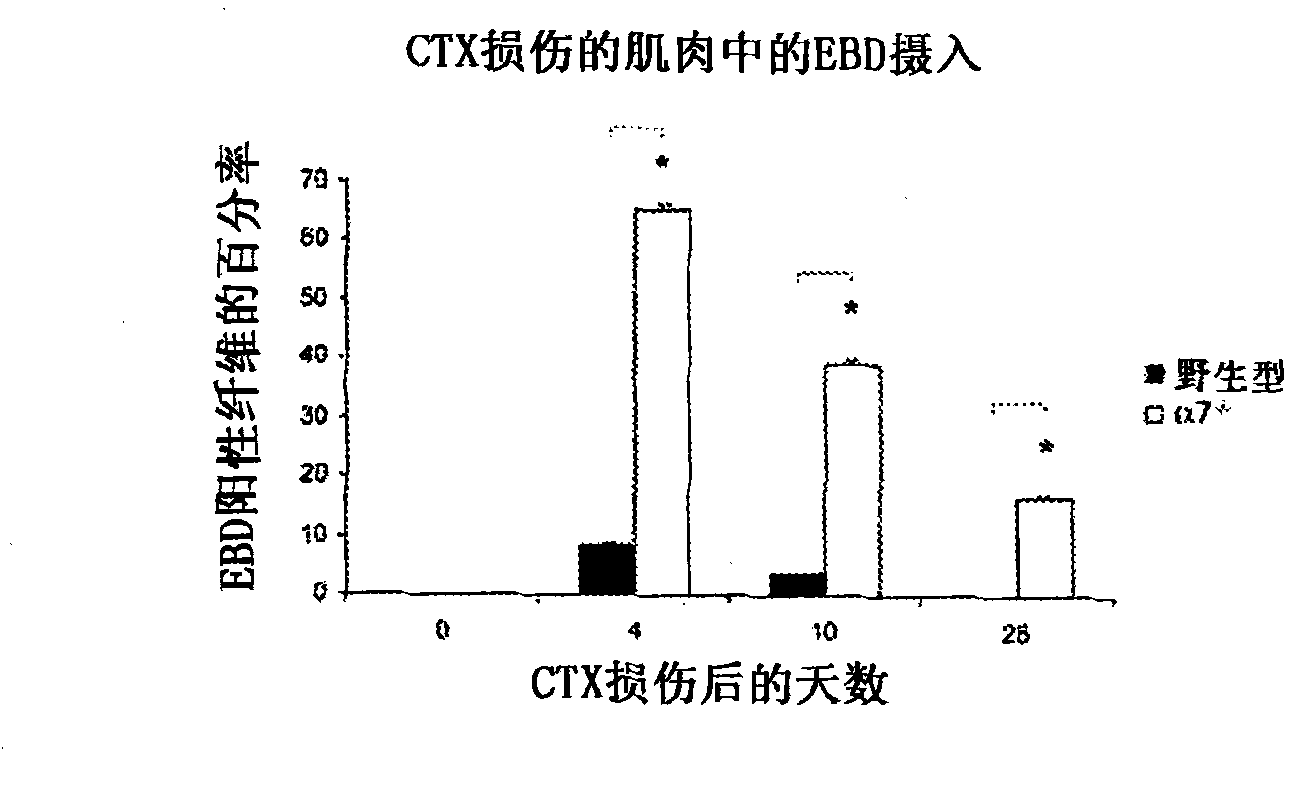
[0162] The tibialis anterior muscle (TA) was embedded in Optimal Cutting Temperature (OCT) (Tissue-Tek; Sakura Finetek, Torrance, California, United States), and using a Leica CM1850 cryostat, 10 μm cryosections (separation ≥ 50 μm ), placed on Surgipath microscope slides (Surgipath Medical Industries, Richmond, IL). As previously reported in Rooney et al., "Severe muscular dystrophy in mice that lack dystrophin and alpha7 intergrin", J. Cell Sci. 119: 2185-2195 (2006 ), which is hereby incorporated by reference to the extent it does not conflict with th...
Embodiment 2
[0220] Materials and Methods
[0221] animal
[0222] In these studies, C57BL / 10ScSn (wild-type) and C57BL / 10ScSn-Dmdmdx / J (mdx) strain mice (Jackson Laboratories, BarHarbor, ME) were used in accordance with the Animal Care and Use Guidelines of the University of Nevada, Reno. Use in animal protocols approved by the Animal Care and Use Committee.
[0223] α7βgal + / - Isolation of myoblasts
[0224] α7βgal from 10 days old + / - The gastrocnemius muscle was removed from the mouse, and the tissue was minced with scissors. Cells were enzymatically dissociated with 1.25 mg / ml type II collagen (Worthington Biochemical Corporation, Lakewood, NJ) at 37°C for 1 hour. The slurry was gently ground and filtered through a nylon mesh. Cells were separated from muscle fiber fragments by differential centrifugation and plated on 100 mm tissue culture plates. Myoblasts were cultured in proliferation medium (Dulbecco's modified Eagle's supplemented with 10% fetal bovine serum (FBS)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com