Preparation method of 2-fluoro-5-bromoxynil
A technology of bromoxynil and o-fluorobromobenzene is applied in the field of preparation of pharmaceutical intermediate 2-fluoro-5-bromobenzonitrile, and achieves the effects of reduced pollution and toxicity, mild reaction conditions and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
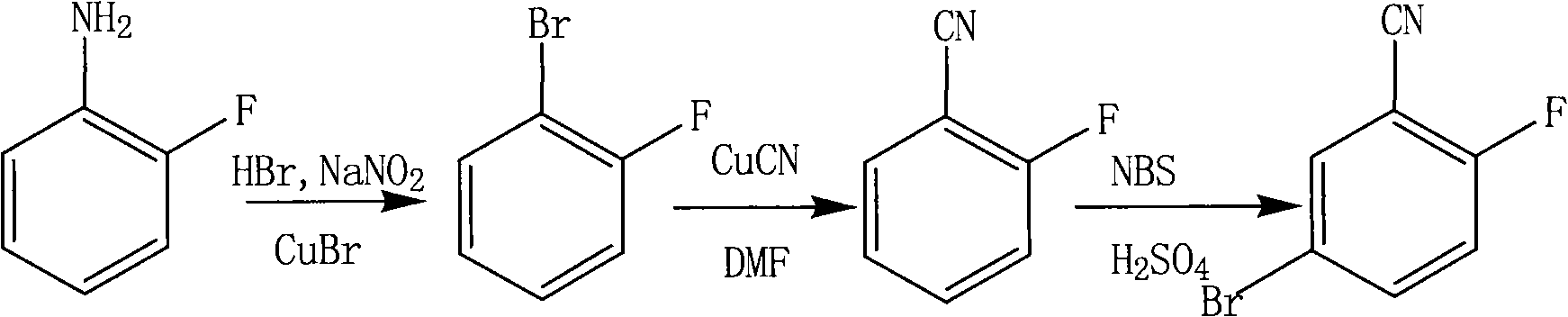
[0013] ① Add 70g 40% HBr, 70ml HBr to a 250ml flask 2 O, 11.1g o-fluoroaniline, heated to 70-80°C for reaction. Cool down to 40-50°C, add 7.2g CuBr, and stir well. Add 8.3g NaNO dropwise 2 , 25ml H 2 O is made into the solution, after dripping, keep warm and react until the end. Cool down to room temperature, pour 200ml H 2 In O, the organic layer was separated, and the organic layer was washed with water to be close to neutral, and anhydrous Na 2 SO 4 Dry, filter with suction, and then distill under reduced pressure to obtain 14.4 g of o-fluorobromobenzene as a light yellow transparent liquid with a yield of 82.8%.
[0014] ②Add 40ml of DMF, 11.7g of CuCN, and 17.4g of o-fluorobromobenzene into a 250ml flask, heat to 150°C, and react for 5h. After the reaction, steam distillation was carried out to obtain 7.26 g of o-fluorobenzonitrile as a yellow transparent liquid with a yield of 60%.
[0015] ③ Add 20ml of concentrated H to a 100ml flask 2 SO 4 , 6.05g o-fluorobe...
Embodiment 2
[0017] ① Add 70g 40% HBr, 70ml HBr to a 250ml flask 2 O, 11.1g o-fluoroaniline, heated to 70-80°C for reaction. Cool down to 40-50°C, add 7.2g CuBr, and stir well. Add 6.9g NaNO dropwise 2 , 21ml H 2 O is made into the solution, after dripping, keep warm and react until the end. Cool down to room temperature, pour 200ml H 2 In O, the organic layer was separated, and the organic layer was washed with water to be close to neutral, and anhydrous Na 2 SO 4 Dry, filter with suction, and then distill under reduced pressure to obtain 10.4 g of o-fluorobromobenzene as a light yellow transparent liquid with a yield of 60%.
[0018] ②Add 40ml of DMF, 11.7g of CuCN, and 17.4g of o-fluorobromobenzene into a 250ml flask, heat to 150°C, and react for 5h. After the reaction, steam distillation was carried out to obtain 7.26 g of o-fluorobenzonitrile as a yellow transparent liquid with a yield of 60%.
[0019] ③ Add 20ml of concentrated H to a 100ml flask 2 SO 4 , 6.05g o-fluorobenz...
Embodiment 3
[0021] ① Add 70g 40% HBr, 70ml HBr to a 250ml flask 2 O, 11.1g o-fluoroaniline, heated to 70-80°C for reaction. Cool down to 40-50°C, add 7.2g CuBr, and stir well. Add 7.6g NaNO dropwise 2 , 23ml H 2 O is made into the solution, after dripping, keep warm and react until the end. Cool down to room temperature, pour 200ml H 2 In O, the organic layer was separated, and the organic layer was washed with water to be close to neutral, and anhydrous Na 2 SO 4 Dry, filter with suction, and then distill under reduced pressure to obtain 12.4 g of o-fluorobromobenzene as a light yellow transparent liquid with a yield of 71%.
[0022]②Add 40ml of DMF, 11.7g of CuCN, and 17.4g of o-fluorobromobenzene into a 250ml flask, heat to 150°C, and react for 5h. After the reaction, steam distillation was carried out to obtain 7.26 g of o-fluorobenzonitrile as a yellow transparent liquid with a yield of 60%.
[0023] ③ Add 20ml of concentrated H to a 100ml flask 2 SO 4 , 6.05g o-fluorobenzo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com