Aromatic solvent free herbicidal formulations of fluroxypyr meptyl ester with C4-C8 esters of triclopyr, 2,4-d or MCPA
A technology of isooctyl fluroxypyr and butoxyethyl triclopyr, which is applied in the field of herbicide preparations and can solve problems such as unoptimized environmental distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
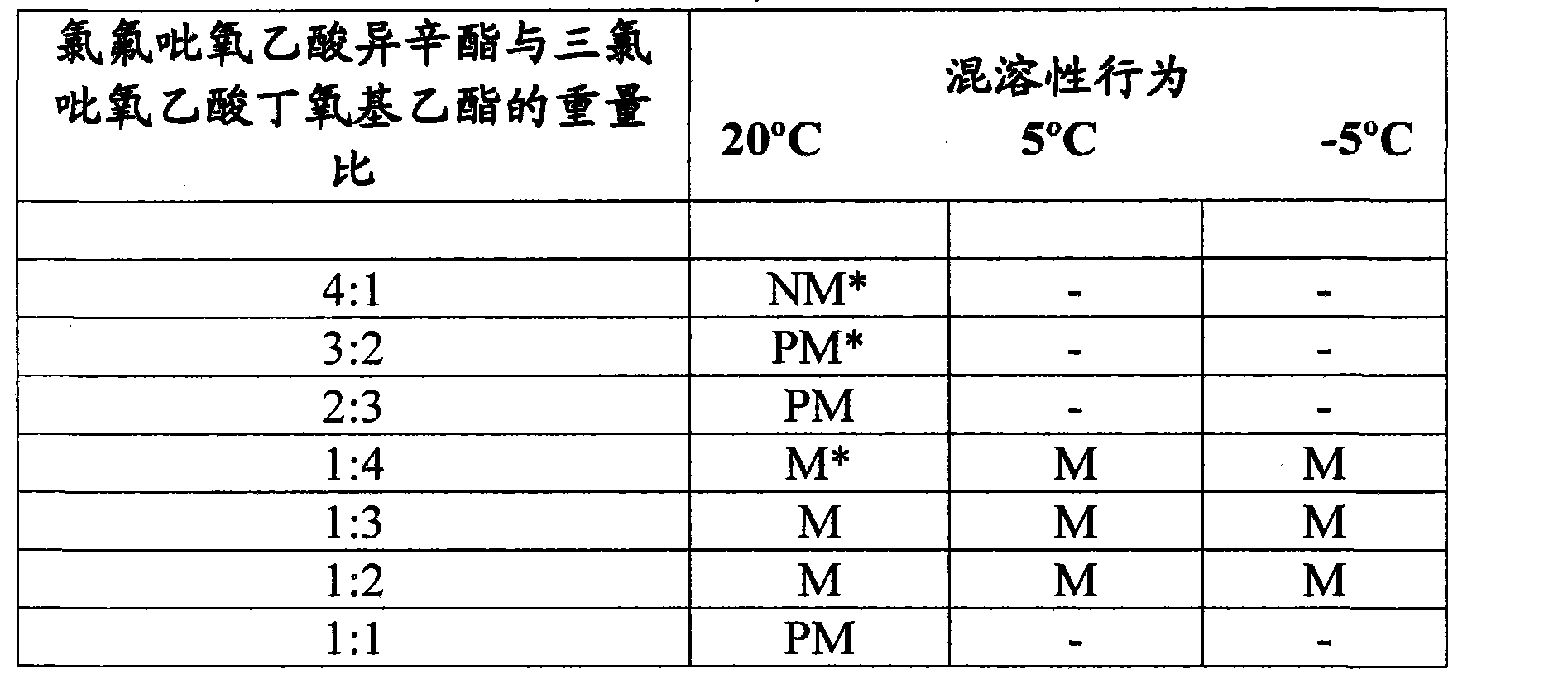
[0012] Example 1: Miscibility of isooctyl chlorofluoropyroxyacetate and butoxyethyl trichloropyroxyacetate ( Miscibility )
[0013] The test system in Table I below was prepared by blending molten isooctyl chlorofluoroacetate (about 65°C) into butoxyethyl chlorofluoroacetate at ambient temperature until isotropic ( isotropic). Miscibility behavior is reported in Table I.
[0014] Table I
[0015]
[0016] * NM = Immiscible
[0017] PM = Partially miscible, crystallisation of isooctyl chlorofluoropyracetate can be observed
[0018] M = miscible, the system is isotropic
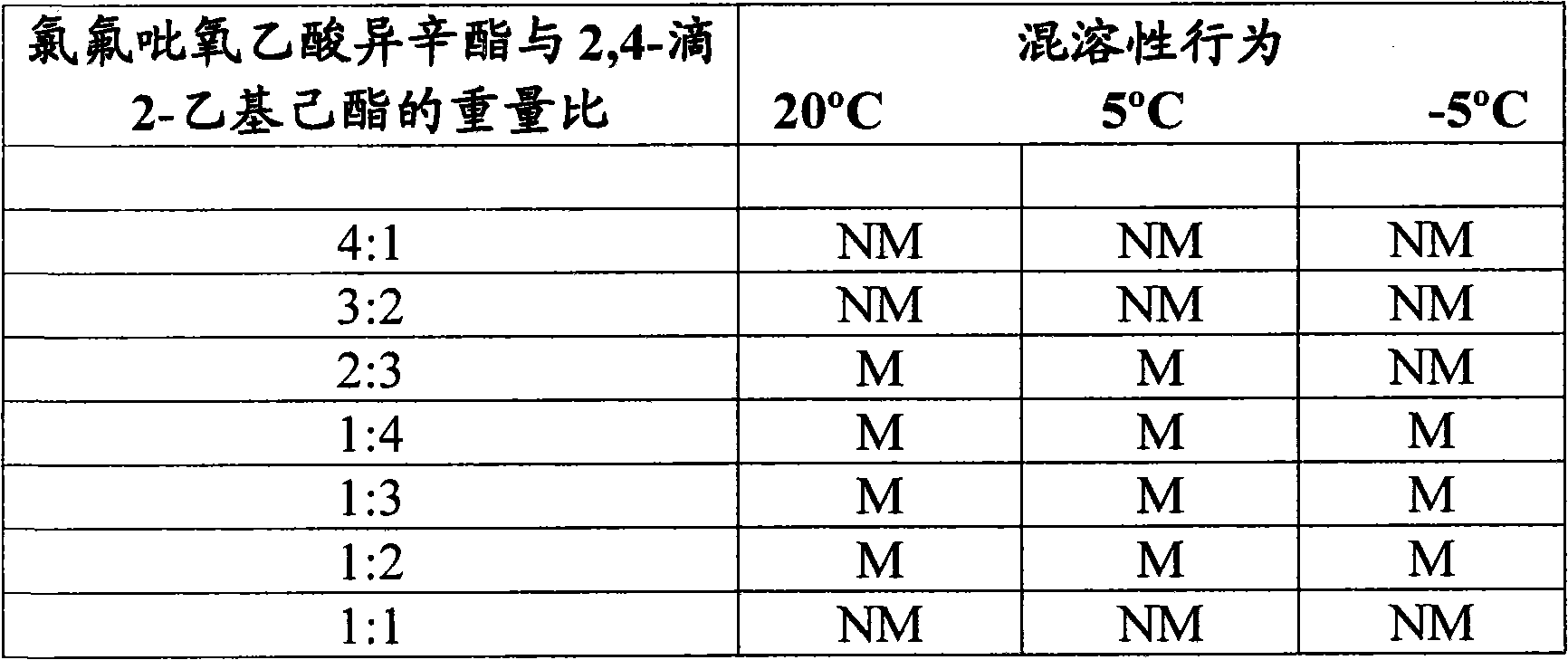
[0019] The test system in Table II below was prepared by blending molten isooctyl chlorofluoropyroxyacetate (about 65°C) into 2,4-D 2-ethylhexyl ester at ambient temperature until isotropic (isotropic). Miscibility behavior is reported in Table II.
[0020] Table II
[0021]
[0022] NM = Immiscible
[0023] PM = Partially miscible, crystallisation of isooctyl chlorofluoropyracetate can be obse...
Embodiment 2
[0031] Example 2: Emulsifiable Concentrate (EC)
[0032] Formulations A and B were prepared as follows. The molten isooctyl chlorofluoroacetate (approximately 65°C) was mixed into the chlorofluoroacetate butoxyethyl ester and the remaining ingredients with stirring at ambient temperature until an isotropic system was obtained.
[0033] Formulation A
[0034] weight%
[0035] Isooctyl chlorofluoropyroxyacetate 22.7
[0036] Butoxyethyl triclosan 65.7
[0037] Agnique BL 2904 (surfactant blend) 11.6
[0038] Formulation B
[0039] weight%
[0040] Isooctyl chlorofluoropyroxyacetate 15.3
[0041] Butoxyethyl triclosan 44.4
[0042] Agnique BL2904 (surfactant blend) 8.0
[0043] Soybean Oil (viscocity modifier) 32.3
Embodiment 3
[0044] Example 3: Emulsion Concentrate in Water (EW)
[0045] Formulation C was prepared as follows. Molten isooctyl chlorofluoroacetate (about 65°C) was mixed with stirring at ambient temperature into a preform of chlorofluoroacetate butoxyethyl, Amisoft HS-21P, Nikkol DGMS, Tween 61 and soybean oil. Blend in the preblend oil phase until isotropic. The mixed oil phase was vigorously mixed with a premixed aqueous phase formed of water, Proxel GXL, sodium dihydrogen phosphate, disodium hydrogen phosphate and propylene glycol until the entire system was homogeneous.
[0046] Formulation C
[0047] Emulsion in Water Concentrate (EW)
[0048] weight%
[0049] Isooctyl chlorofluoropyroxyacetate 7.9
[0050] Butoxyethyl triclosan 22.9
[0051] Amisoft HS-21P (surfactant) 0.5
[0052] Nikkol DGMS (surfactant) 2.0
[0053] Tween 61 (surfactant) 1.4
[0054] Soybean oil (viscosity modifier) 10.0
[0055] Proxel GXL (biocide) 0.3
[0056] NaH 2 PO 4 ·H 2 O (buffer) 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com