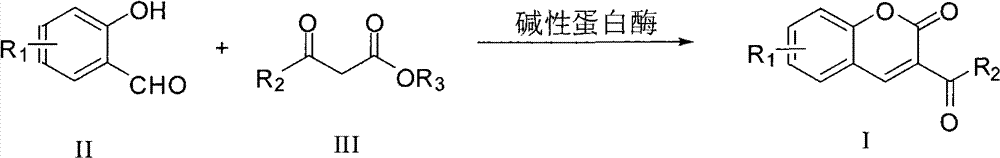
Synthesis method of 2H-1-benzopyran-2-ketone derivatives
A technology of benzopyran and synthesis method, which is applied in the field of synthesis of 2H-1-benzopyran-2-one derivatives, can solve the problems of cumbersome operation, severe reaction conditions, large amount of catalyst, etc., and achieve chemical selection High stability, high product yield and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~35
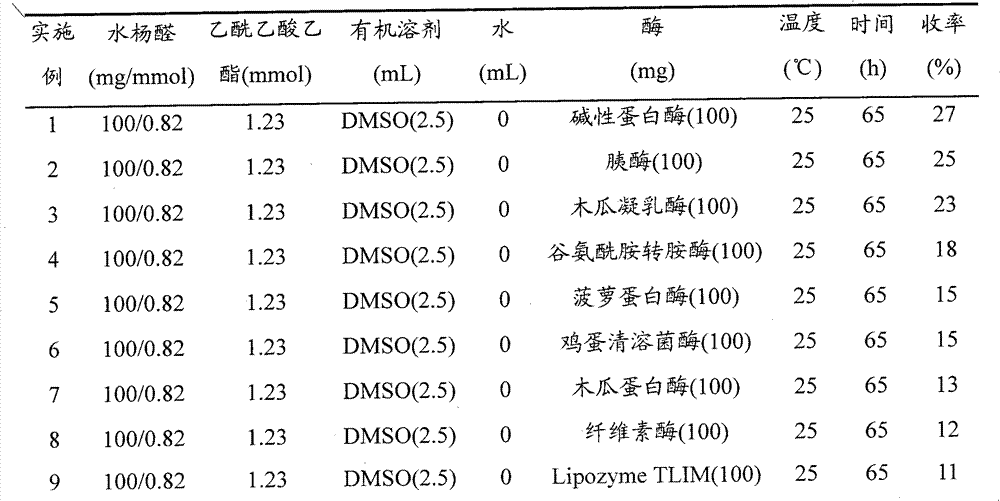
[0017] Examples 1-35. Synthesis of 3-acetyl-2H-1-benzopyran-2-one by enzyme-catalyzed dominoic / intramolecular transesterification of salicylaldehyde and ethyl acetoacetate
[0018]
[0019] Add salicylaldehyde, ethyl acetoacetate, organic solvent, water and enzyme in the round bottom flask, constant temperature stirring reaction, after the completion of the reaction, filter, the filter cake is washed with dichloromethane, the combined filtrate and lotion, add water (20ml ) after dilution, extracted 3 times (each 20ml) with dichloromethane, combined the dichloromethane extracts, dried with anhydrous sodium sulfate, then removed the solvent by distillation under reduced pressure to obtain the crude product, and the crude product was purified by flash column chromatography , the target product is obtained. The reaction conditions and results are shown in Table 1.
[0020] Table 1, Synthesis conditions and results of 3-acetyl-2H-1-benzopyran-2-one
[0021]
[0022]
[0...
Embodiment 36~49
[0024] Examples 36-49, Synthesis of 2H-1-benzopyran-2-one derivatives by alkaline protease-catalyzed salicylaldehyde or its derivatives and β-keto ester dominoic culture / intramolecular transesterification reaction
[0025]
[0026] Add salicylaldehyde or its derivatives (1.64mmol), β-ketoester (4.9mmol), DMSO (4.5mL), water (0.5mL) and alkaline protease (100mg) in a round bottom flask, temperature 55°C Stir the reaction, after the reaction is complete, filter, the filter cake is washed with dichloromethane, combine the filtrate and washings, add water (20ml) to dilute, extract 3 times with dichloromethane (each 20ml), and combine the dichloromethane extracts , dried with anhydrous sodium sulfate, and then distilled off the solvent under reduced pressure to obtain a crude product, which was purified by flash column chromatography to obtain 2H-1-benzopyran-2-one derivatives. The reaction conditions and results are shown in Table 2.
[0027] Table 2, Synthetic conditions and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com