Artificially synthesized signal peptide and application thereof
A signal peptide, mammalian technology, applied in the direction of peptides, introduction of foreign genetic material using a carrier, and cells modified by introduction of foreign genetic material, etc., can solve the problems of low expression efficiency and high cost of foreign protein expression in animal cells, and achieve the The effect of increasing protein expression, increasing expression level and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
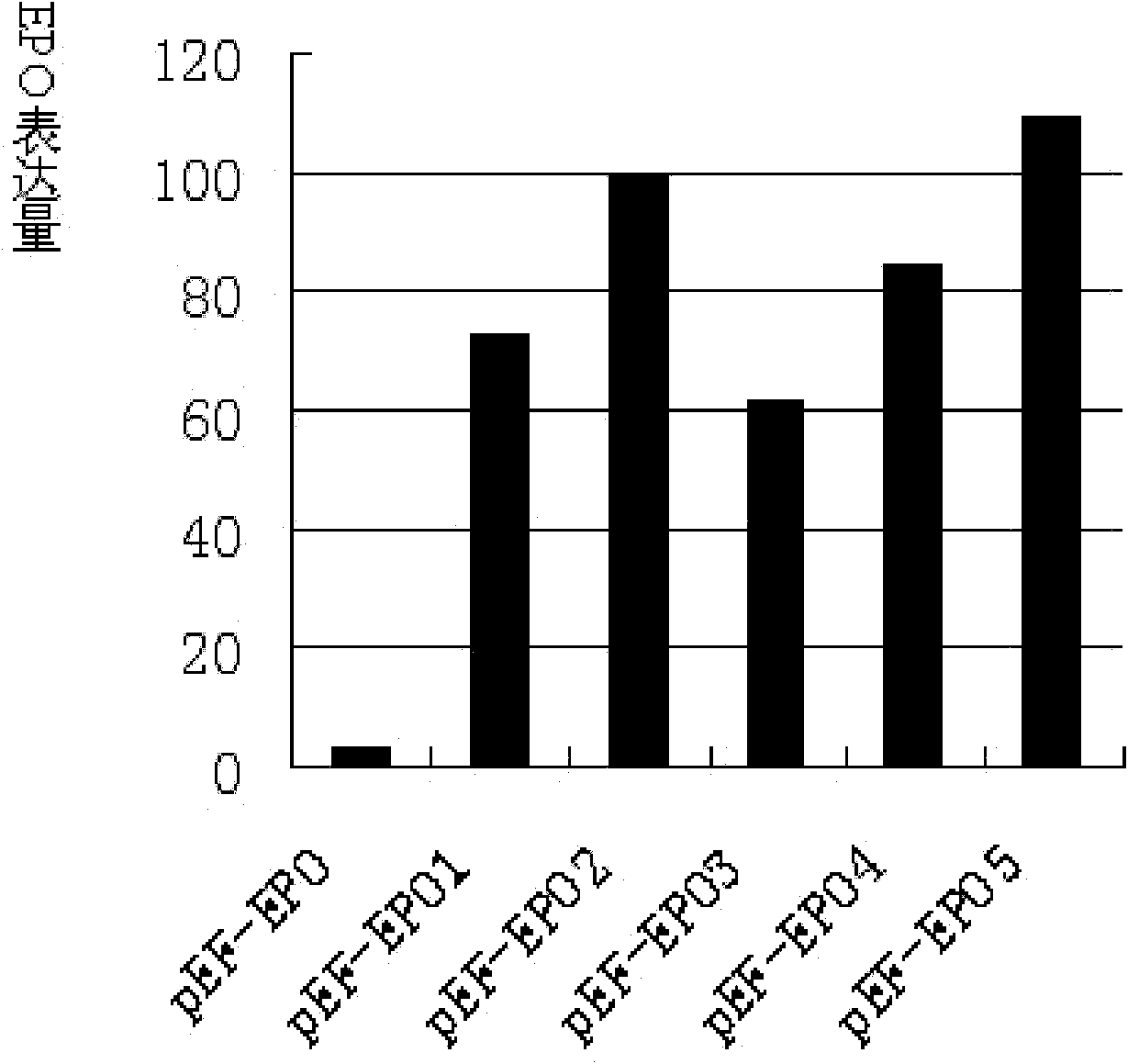
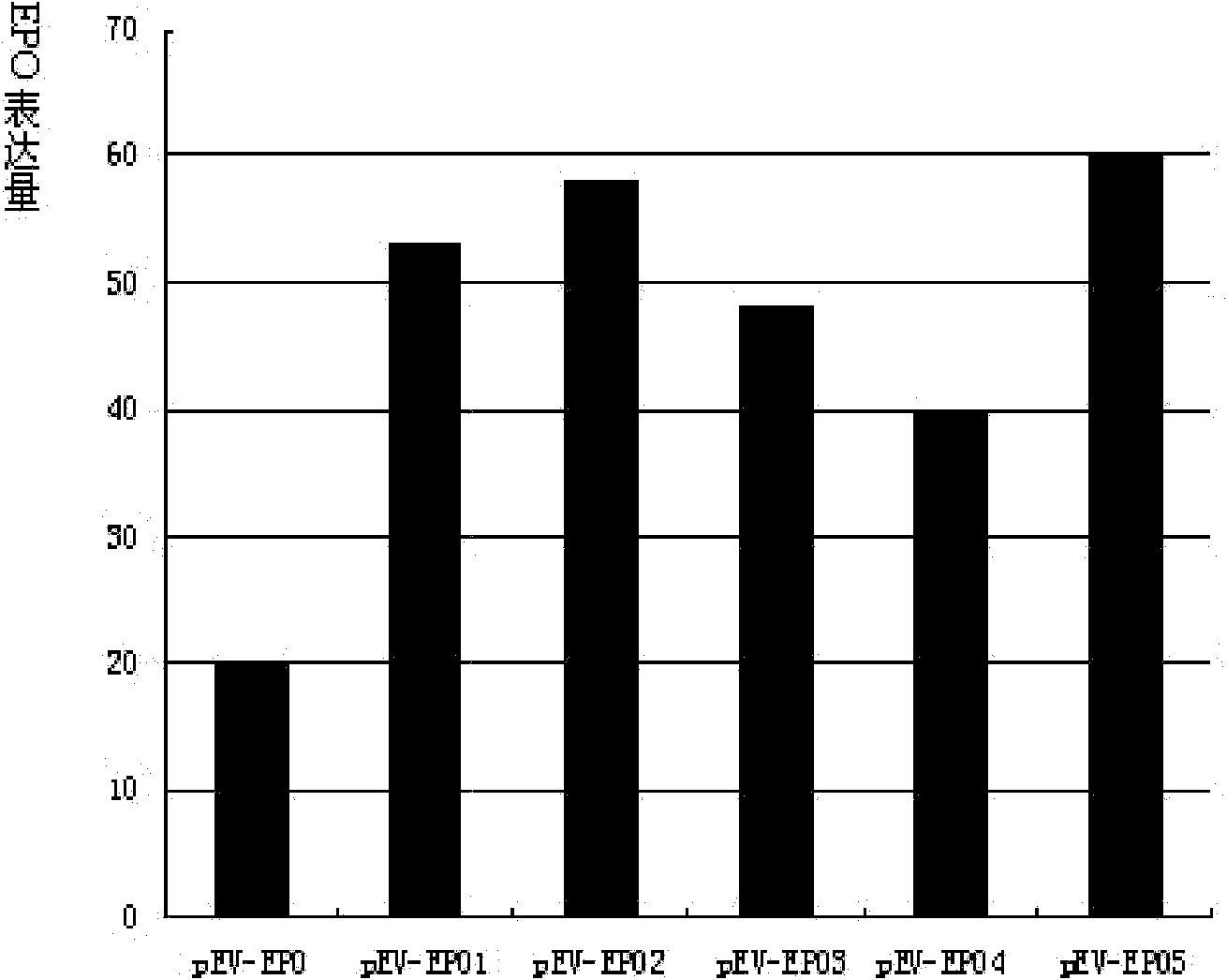
Image
Examples
Embodiment 1
[0044] Example 1 Synthesis of Signal Peptide
[0045] Because the signal peptide is short, the signal peptide is synthesized according to the primer. The NheI restriction site and Kozak sequence are added to the 5' end, and the target gene sequence is added to the 3' end, so as to add the signal peptide on the target gene.
[0046] Signal peptide 1: TCGGAGCTAGCCACC ATGGATGTTCTTGCTTTTCTTCTGGGCTTGCTGCTTTTGTGGCTTCCCGGGGTGAGGTGC GCCCCACCACG (SEQ ID NO: 11)
[0047] Signal peptide 2: TCGGAGCTAGCCACC ATGGATGTTCCTGCCGAATTTCTTGGATTGTTGTTGTTGTGGCTCTCCGGAGTGCGTTGC GCCCCACCACG (SEQ ID NO: 12)
[0048] Signal peptide 3: TCGGAGCTAGCCACC ATGAGGGTTCTGCCTGAATTCCTGGGACTTTTGTTGTTGTGGATTTCCGGCGTGCGATGT GCCCCACCACG (SEQ ID NO: 13)
[0049] Signal peptide 4: TCGGAGCTAGCCACC ATGGATGTACCACTTCAGCTTCTTGGCTTGTTGTTGCTTTGGCTTTCTGGCGTGAGATGT GCCCCACCACG (SEQ ID NO: 14)
[0050] Signal peptide 5: TCGGAGCTAGCCACC ATGGATGTGCCTGCTGAACTTTTGGGCCTTCTTTTGTTGTGGATATCAGGAGTACGATGC GCCCCACCACG (...
Embodiment 2
[0051] Embodiment 2 Amplification of the human EF-1α promoter
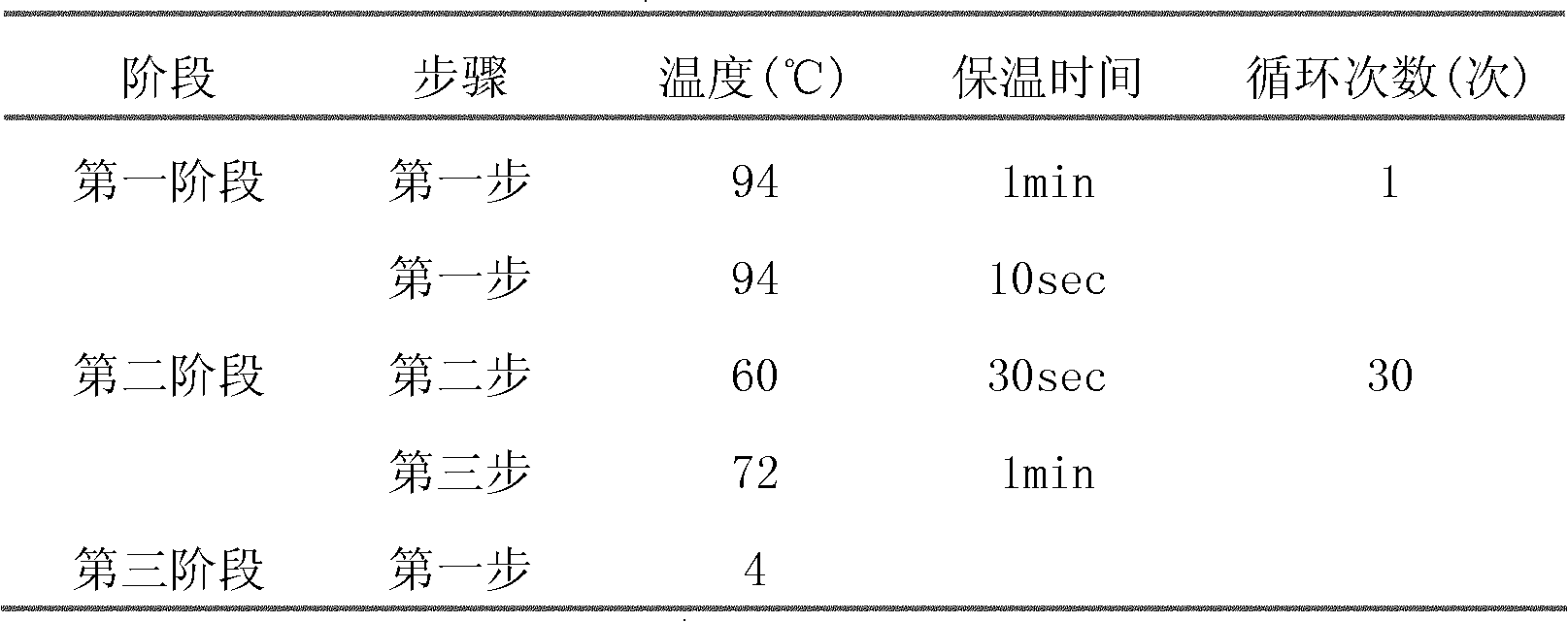
[0052] Using the plasmid pEF6 / V5-HisA (purchased from Invitrogen) as a template and the human EF-1α promoter sequence as a reference, primers F01 / R01 were designed and polymerase chain reaction was performed to amplify the human EF-1α promoter sequence, The reaction conditions are shown in Table 1.
[0053] F01: CATACTAGTGCTCCGGTGCCCGTCAGTGGGCAGAG (SEQ ID NO: 16)
[0054] R01: ACGGCTAGCTCCGAGCTCGGTACCAAGCTTACCTAGCCA (SEQ ID NO: 17)
[0055] Table 1 PCR reaction conditions
[0056]
[0057] The resulting PCR product was ligated with SmaI-treated pUC57 (purchased from Fermentas), and sequenced for identification. The results showed that the sequence was as follows:
[0058] ACTAGTGCTCCGGTGCCCGTCAGTGGGCAGAGCGCACATCGCCCACAGTCCCCGAGAAGTTGGGGGGAGGGGTCGGCAATTGAACCGGTGCCTAGAGAAGGTGGCGCGGGGTAAACTGGGAAAGTGATGTCGTGTACTGGCTCCGCCTTTTTCCCGAGGGTGGGGGAGAACCGTATATAAGTGCAGTAGTCGCCGTGAACGTTCTTTTTCGCAACGGGTTTGCCGCCAGAACACAGGTAA...
Embodiment 3
[0059] Embodiment 3 Amplification of the human EF1-HTLV promoter
[0060] Using the plasmid pFUSE-CHIg-hG3 (purchased from InvivoGen) as a template and the human EF1-HTLV promoter sequence as a reference, primers F02 / R02 were designed and polymerase chain reaction was performed to amplify the human EF1-HTLV promoter sequence, The reaction conditions are shown in Table 1.
[0061] F02: ACGACTAGTGCTCCGGTGCCCGTCAGTGGGCAGAGC (SEQ ID NO: 19)
[0062] R02: ATCGCTAGCGTAGGCGCCGGTCACAGCT (SEQ ID NO: 20)
[0063] The resulting PCR product was ligated with SmaI-treated pUC57 (purchased from Fermentas), and sequenced for identification. The results showed that the sequence was as follows:
[0064] ACTAGTGCTCCGGTGCCCGTCAGTGGGCAGAGCGCACATCGCCCACAGTCCCCGAGAAGTTGGGGGGAGGGGTCGGCAATTGAACGGGTGCCTAGAGAAGGTGGCGCGGGGTAAACTGGGAAAGTGATGTCGTGTACTGGCTCCGCCTTTTTCCCGAGGGTGGGGGAGAACCGTATATAAGTGCAGTAGTCGCCGTGAACGTTCTTTTTCGCAACGGGTTTGCCGCCAGAACACAGCTGAAGCTTCGAGGGGCTCGCATCTCTCCTTCACGCGCCCGCCGCCCTACCTGAGGCC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com