Cardiovascular/cerebral and ophthalmological medicines, and preparation and use thereof
A cardio-cerebrovascular and ophthalmology technology, applied in the field of cardio-cerebrovascular and ophthalmic drugs and their preparation, to achieve the effect of easy storage and transportation, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
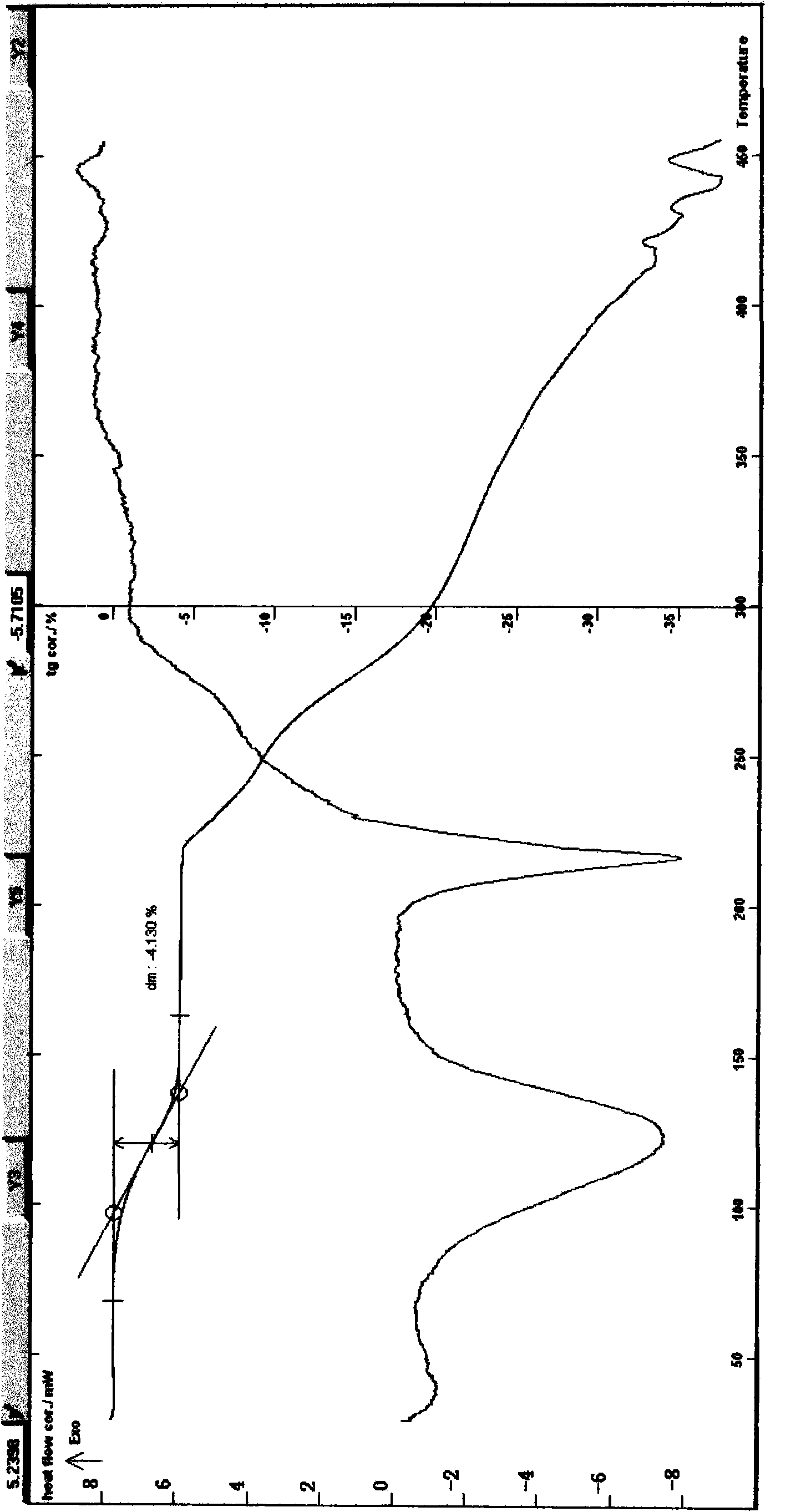
[0048] Example 1 Preparation of Puerarin Monohydrate In a reaction vessel, 80 g of dried Pueraria root powder was extracted 3 times with 400 ml of ethanol 80% aqueous solution, and the extracts were combined, coarsely filtered, and then filtered with a 0.22 μm microporous membrane, and the filtrate was concentrated , to obtain Pueraria root extract, add water, adjust PH=3-5 with dilute hydrochloric acid, then adjust PH between 6-7 with sodium bicarbonate solution, filter, then use n-butanol and isohexanone as solvents to extract, and the extract is evaporated Dry to obtain a solid, use water, methanol, formic acid, and acetonitrile as crystallization solvents, recrystallize three times, filter, wash with water, and dry the solid at about 80°C for 4 hours to obtain 1.8g of off-white crystalline powder; melting point: 208.4~212.5°C decomposition (uncorrected), UV spectrum: λ CH3OH max 250nm, [a] D 21 +18.14°C (c=1, methanol), ESI-MS: m / z: 417[M+1] + ;Infrared Spectrum: v KB...
Embodiment 2
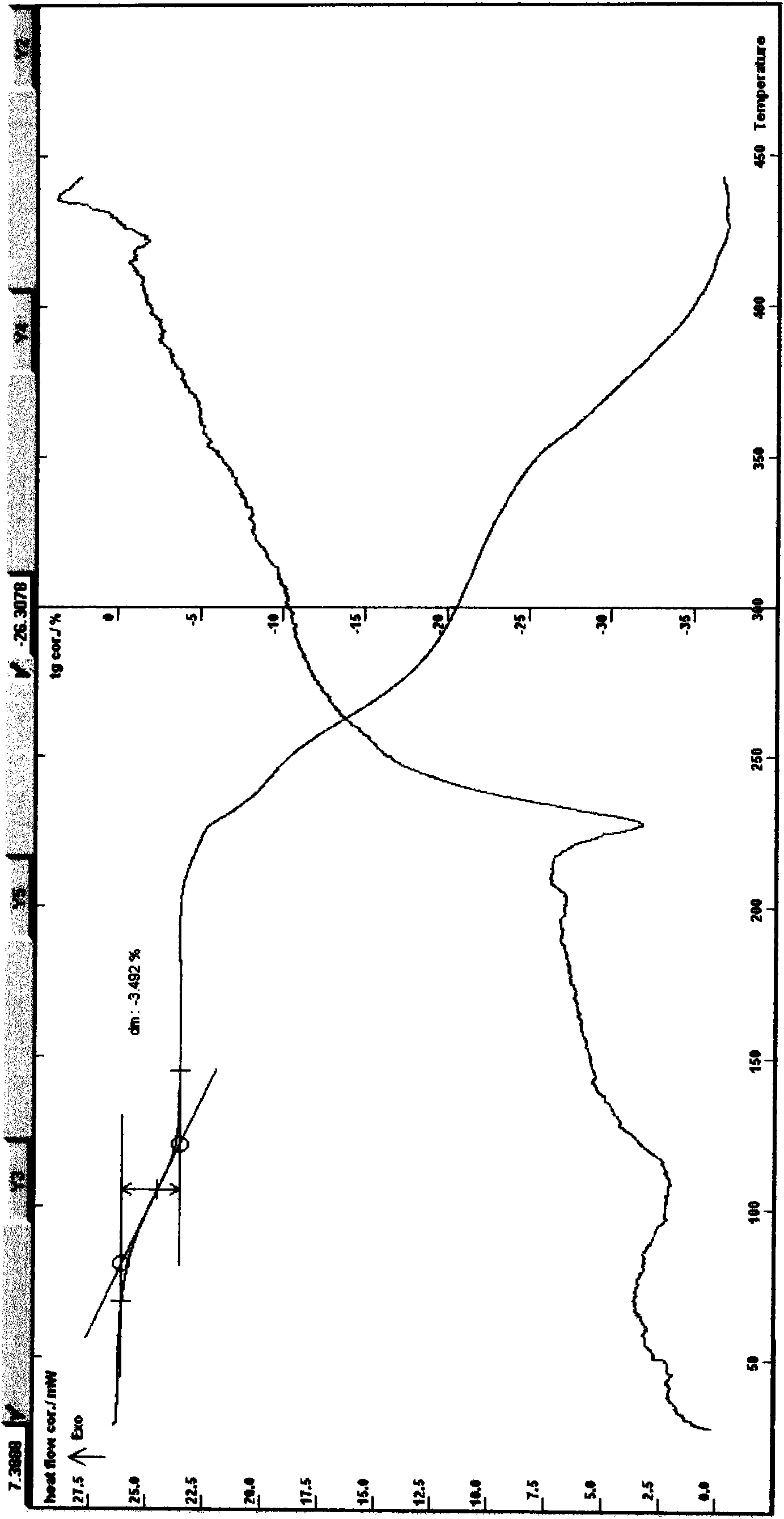
[0049] Example 2 Preparation of Puerarin Crystal Hydrate Add 30 g of puerarin extract or total flavonoids of puerarin, add water, heat to about 50-70°C, filter through a 0.15-0.24 μm ceramic membrane, pass the filtrate through a neutral alumina chromatography column, and elute , D101 macroporous resin adsorption, washing with water, TLC monitoring, and then eluting with 30-75% ethanol aqueous solution until the elution is complete, filtering, concentrating under reduced pressure, standing still, using water, acetone, ethanol, acetonitrile as crystals Solvent, recrystallized twice, left to stand, filtered, washed with water, and dried the obtained solid; dried at about 80°C for 4-6 hours to obtain 6.6 grams of off-white solid, melting point: 227.8-231.8°C (uncorrected), UV spectrum: lambda CH3OH max 250nm; [a] D 21 +18.14°C (c=1, methanol), ESI-MS: m / z: 417[M+1] + ;Infrared Spectrum: v KBr max cm -1 3380, 1626, 1586, 1516, 1446; Karl Fischer's method measures moisture t...
Embodiment 3
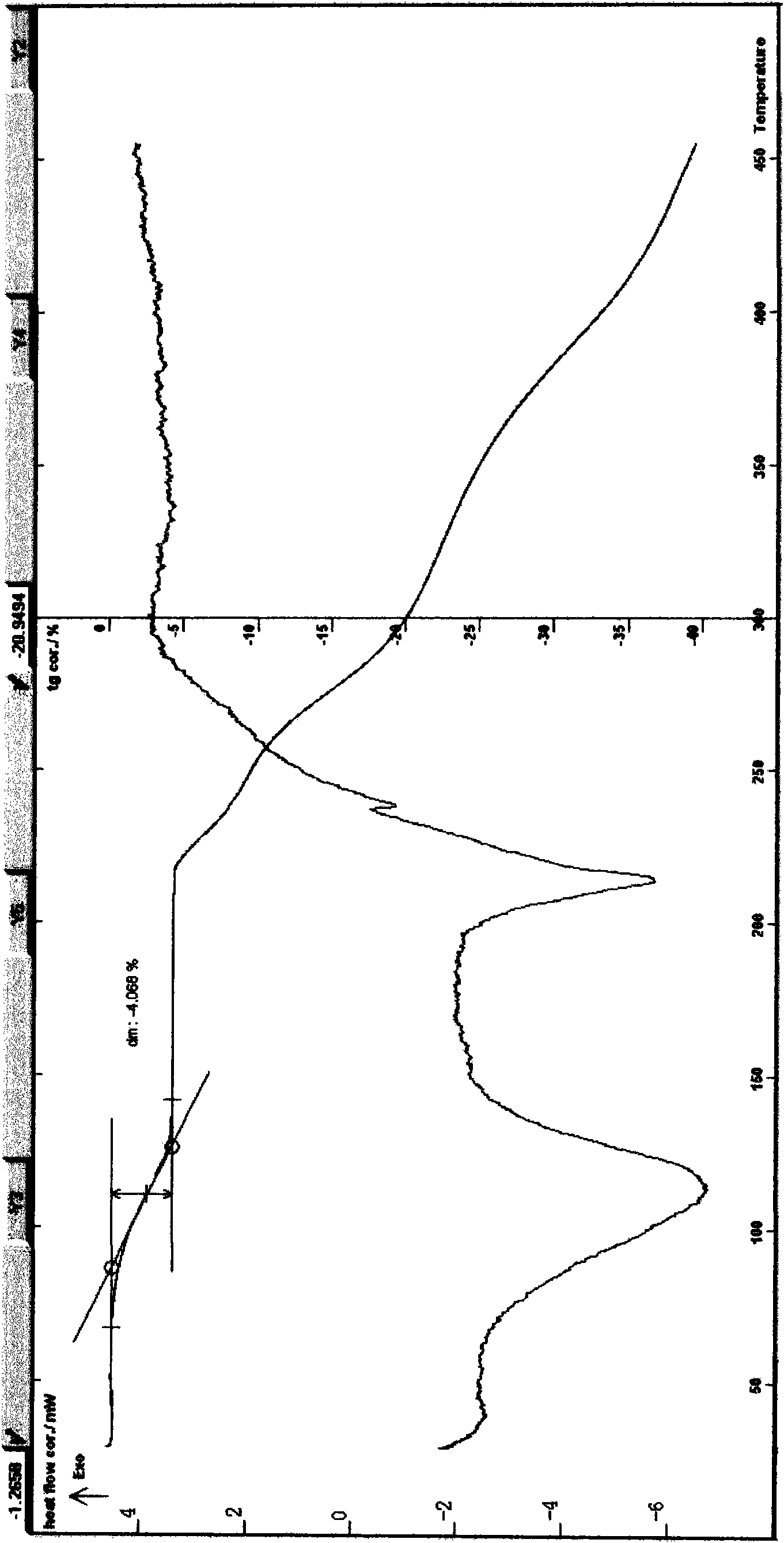
[0050] Example 3 Preparation of Puerarin Monohydrate 90g of dried kudzu root powder of about 40 mesh, add 400ml of water, ultrasonically and reflux extraction 3 times, combine the extracts, coarse filter, and then filter through a 0.22 μm microporous membrane or ceramic membrane, The filtrate is then between 25-65°C, under 0.15-2MPa (the pressure can be dynamically adjusted, or the pressure difference can be increased as the test progresses), and an ultrafiltration membrane (polysulfone hollow fiber membrane module with a molecular weight cut-off of 4000-50000) is used , fiber pore diameter 0.011μm, internal diameter is 1.1mm) filtration, the filtrate is filtered through the nanofiltration membrane with a molecular weight cut-off of more than 200, concentrated, dissolved with 90% methanol aqueous solution, add acetic acid and acetonitrile as the crystallization solvent, place, crystallize, pump Filter and wash with water; as above, recrystallize 3 times with methanol, water, ac...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap