Method for carrying out full quality control on Chinese patent drugs by using mixed contrast
A technology of mixed control and Chinese patent medicine, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of difficult promotion and poor reproducibility, and achieve the effects of saving inspection time and cost, easy promotion and application, and large amount of chromatographic information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
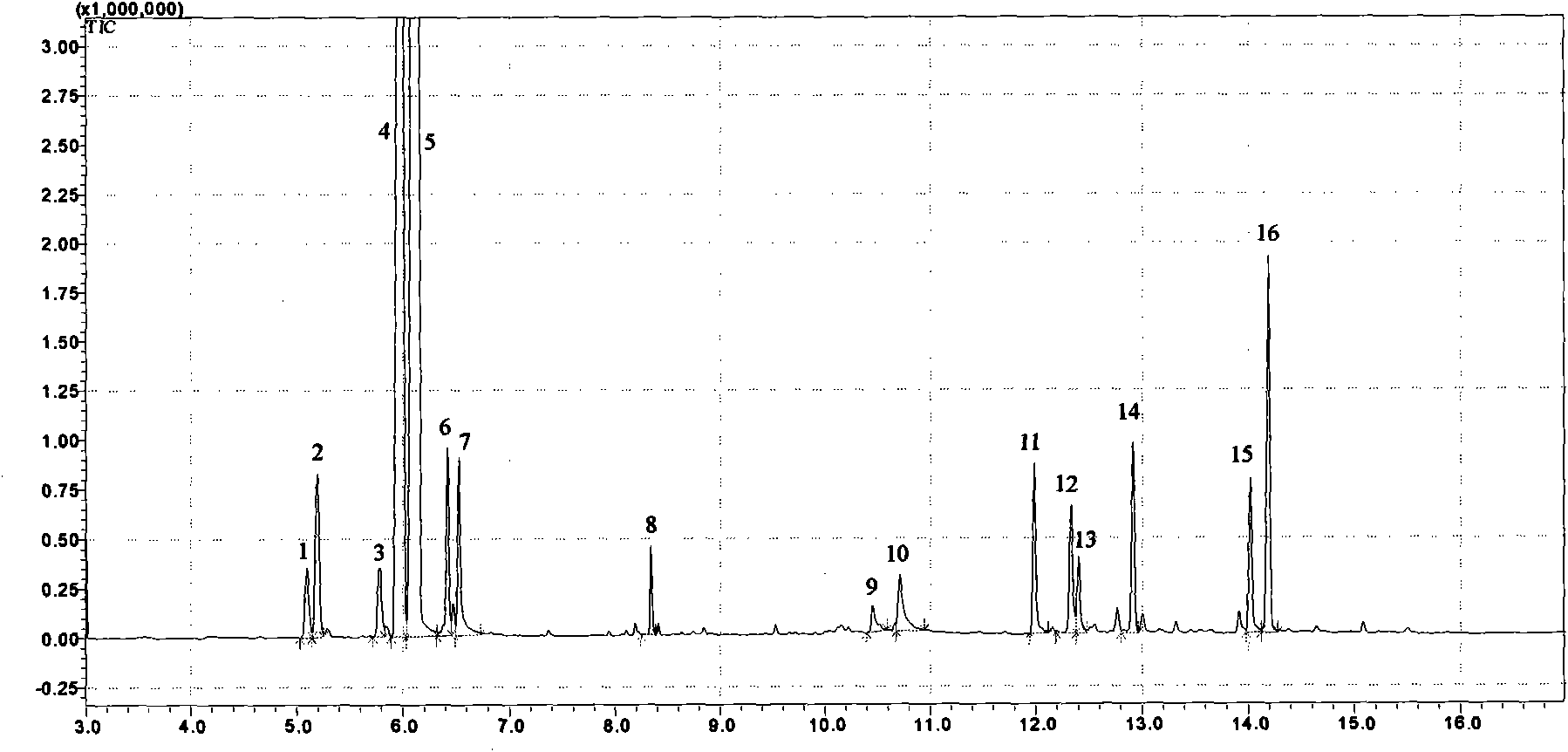
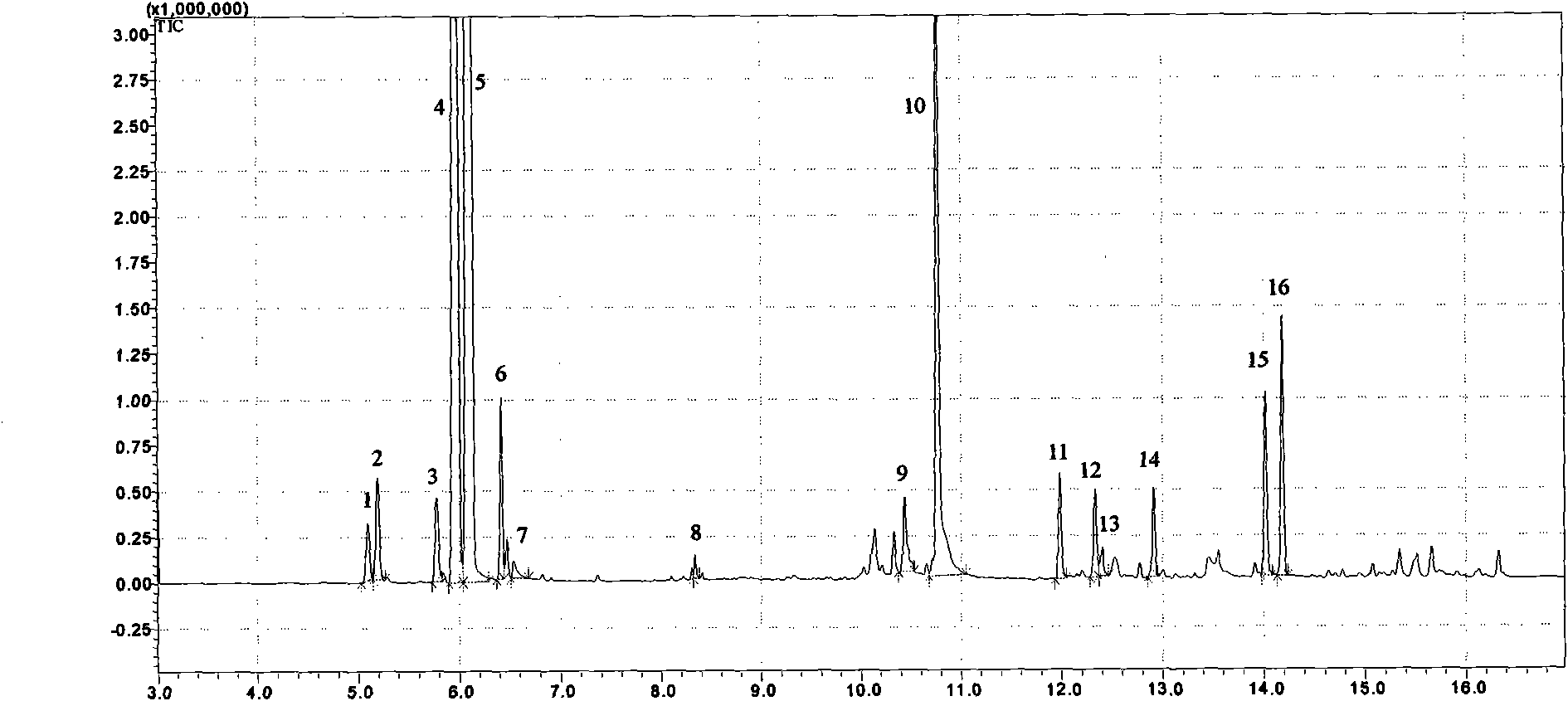
[0028] Embodiment 1 (using gas chromatography-mass spectrometry to identify each medicinal taste in Guanxin Suhe Pills):
[0029] Guanxin Suhe Pills are composed of borneol, frankincense, sandalwood, styrax, and inulin. It has the effects of regulating qi, widening the chest, and relieving pain. It is clinically used for the treatment of angina pectoris, chest tightness, and shortness of breath.
[0030] (1) Main instruments: gas chromatography mass spectrometry (Shimadzu 2010Plus, Japan), balance (Metters AE-163, Switzerland).
[0031] (2) Materials and reagents: borneol, frankincense, sandalwood, styrax, and inulin as reference medicinal materials (National Inspection Institute, batch number: 110743-200504, 120970-200604, 121240-200402, 120931-200202, 121090-200602).
[0032] The test product Guanxin Suhe Pill was purchased from a pharmacy.
[0033] Water is pure water, and other reagents are analytically pure.
[0034] (3) Chromatographic conditions: Chromatographic colum...
Embodiment 2
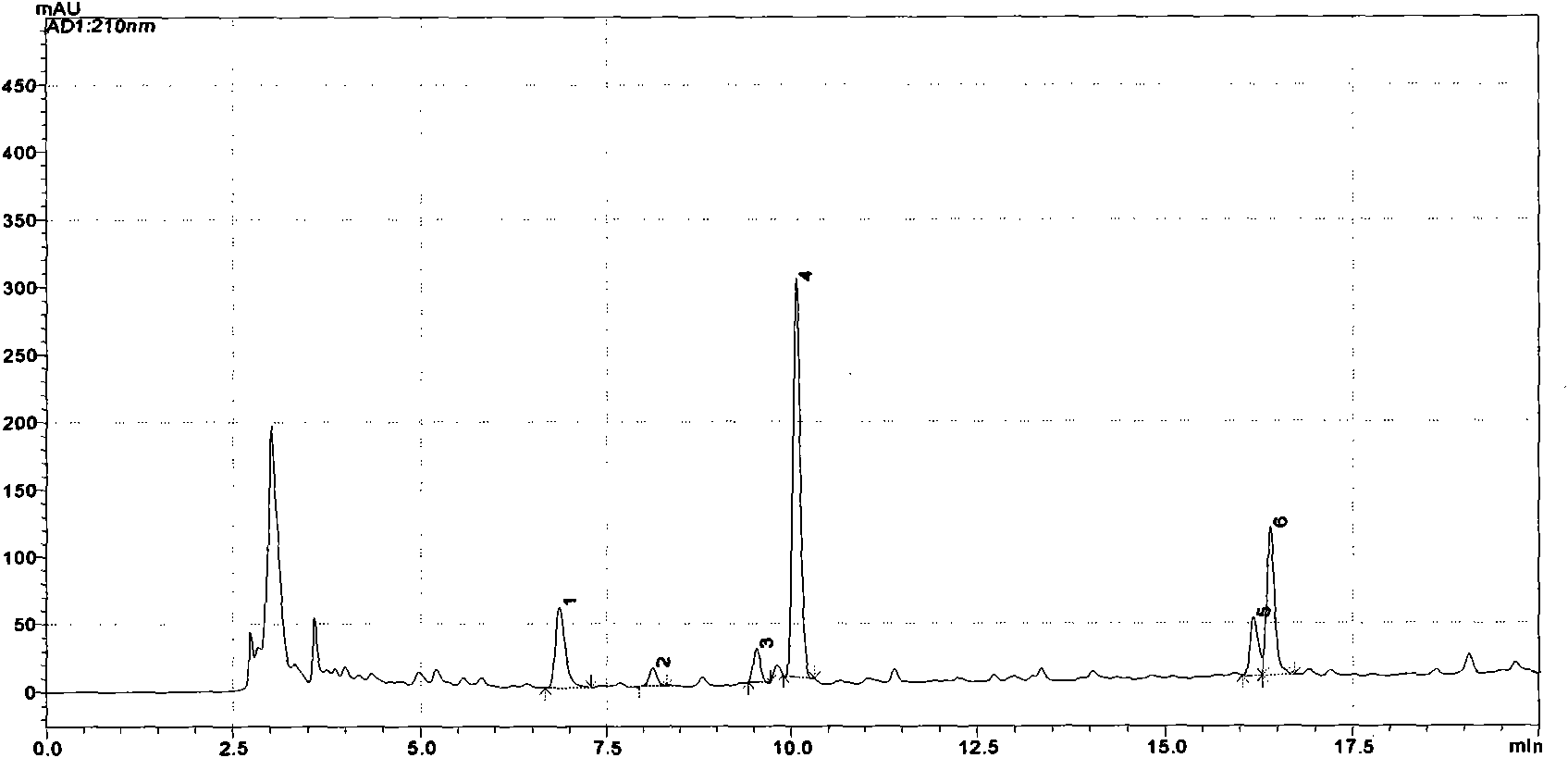
[0044] Example 2: Using high performance liquid chromatography to identify the flavors of each medicine in Zhisou Dingchuan Pills.
[0045] Zhisou Dingchuan Pill is composed of ephedra, bitter almond, licorice, and gypsum. It has the effect of cooling the lungs and relieving asthma. It is mainly used for external cold and internal heat, body heat and thirst, cough with phlegm, and asthma. The treatment of shortness of breath, chest and diaphragm fullness, and acute bronchitis. The gypsum in this prescription is a mineral medicine and has no chromatographic behavior, so its quality control is carried out by microscopic identification, and the quality control of other three herbs such as ephedra, bitter almond, and licorice is carried out by high performance liquid chromatography.
[0046] (1) Main instruments
[0047] High performance liquid chromatography (Shimadzu 20A, Japan), balance (Metters AE-163, Switzerland).
[0048] (2) Materials and reagents
[0049] Ephedra, bitt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com