Synthetic method of 2-(4-alkyl substituted benzoyl) phenylformic acid
A technology of benzoyl and benzoic acid, which is applied in the field of synthesis of 2-(4-alkyl-substituted benzoyl) benzoic acid, can solve the problems of large amount of catalyst, large amount of hydrogen chloride, unfriendly environment, etc. The effect of less waste and low price
Active Publication Date: 2011-01-19
长兴宜生药物科技有限公司
View PDF2 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This method has problems such as a large amount of catalyst used, cannot be recycled, and a large amount of hydrogen chloride is generated after post-processing, which has great corrosion on equipment and is not environmentally friendly.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
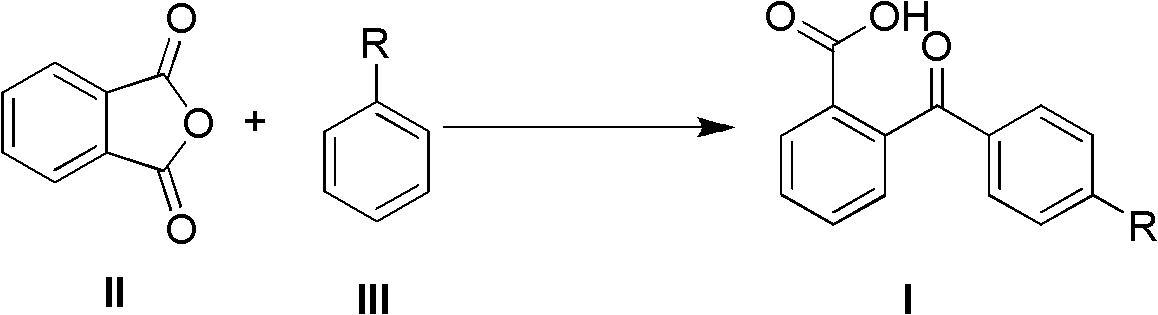
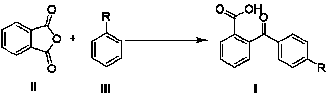
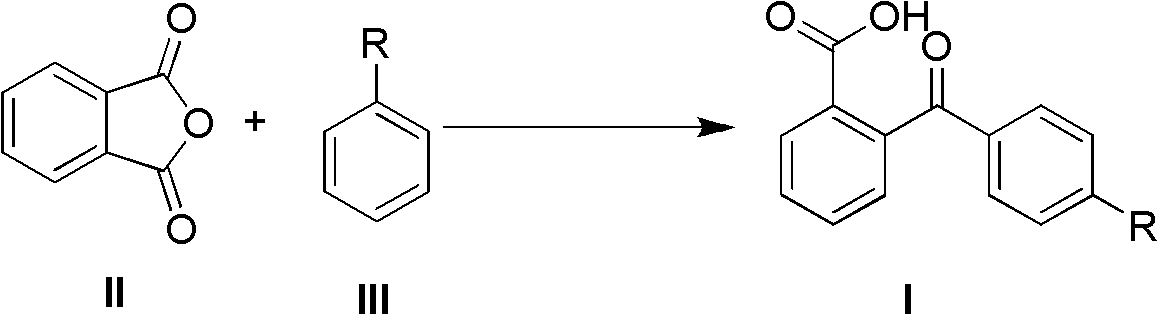
The invention discloses a synthetic method of 2-(4-alkyl substituted benzoyl) phenylformic acid with a structure represented by a formula (I). The 2-(4-alkyl substituted benzoyl) phenylformic acid is prepared by reacting phthalic anhydride with a structure represented by a formula (II) with alkylbenzene with a structure represented by a formula (III) in a reaction solvent, wherein the phthalic anhydride and the alkylbenzene are used as raw materials. The synthetic method is characterized in that trifluoromethanesulfonic salt is used as a catalyst; and a feeding mol ratio of the organic amine trifluoromethanesulfonic salt to the phthalic anhydride is 1-50% :1. The organic amine trifluoromethanesulfonic salt catalyst is used to replace the traditional alchlor catalyst, and the organic aminetrifluoromethanesulfonic salt has high water solubility, water stability and lower cost and is easy to obtain and prepare. The method has the advantages of greatly reduced consumption of the organic amine trifluoromethanesulfonic salt, no waste acid generation, high reaction yield, mild reaction condition, safe and simple operation, few three wastes and larger implementation value.
Description
(1) Technical field The invention relates to a method for synthesizing 2-(4-alkyl-substituted benzoyl)benzoic acid by using phthalic anhydride and alkyl-substituted benzenes as raw materials. (2) Background technology 2-(4-Alkyl-substituted benzoyl)benzoic acid is an important key intermediate in organic synthesis. For example, 2-(4-alkyl substituted benzoyl) benzoic acid is an important intermediate for the synthesis of substituted anthraquinones. 2-Ethylanthraquinone (2-pentylanthraquinone) is the main raw material and intermediate for the production of hydrogen peroxide, photosensitive materials, dyes and degradable resins by anthraquinone method. Before the present invention provides, the industrialized application method of existing literature report synthesis 2-(4-alkyl substituted benzoyl) benzoic acid is (EP 451714,1991, 90%; Journal of Jiangxi Normal University, 2000,24 (2) , 152-154, 85%): The target product is prepared by using phthalic anhydride and alkylben...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C65/34C07C51/083
Inventor 李坚军苏为科施湘君
Owner 长兴宜生药物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com