Glycosylated glycopeptide antibiotic derivative
一种糖肽、化合物的技术,应用在抗菌素、抗菌素、肽等方向,能够解决没有记载氨基化学修饰化合物等问题
Inactive Publication Date: 2011-01-26
SHIONOGI & CO LTD
View PDF37 Cites 14 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Among them, Non-Patent Document 11 (Angewandte Chemie, International Edition (2003), 42(38), 4657-4660) describes that -CH is bonded to galactose at the end of the sugar chain 2 NH 2 vancomycin derivatives, but there is no record of compounds that further chemically modify the amino group
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment 13
Embodiment 30
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
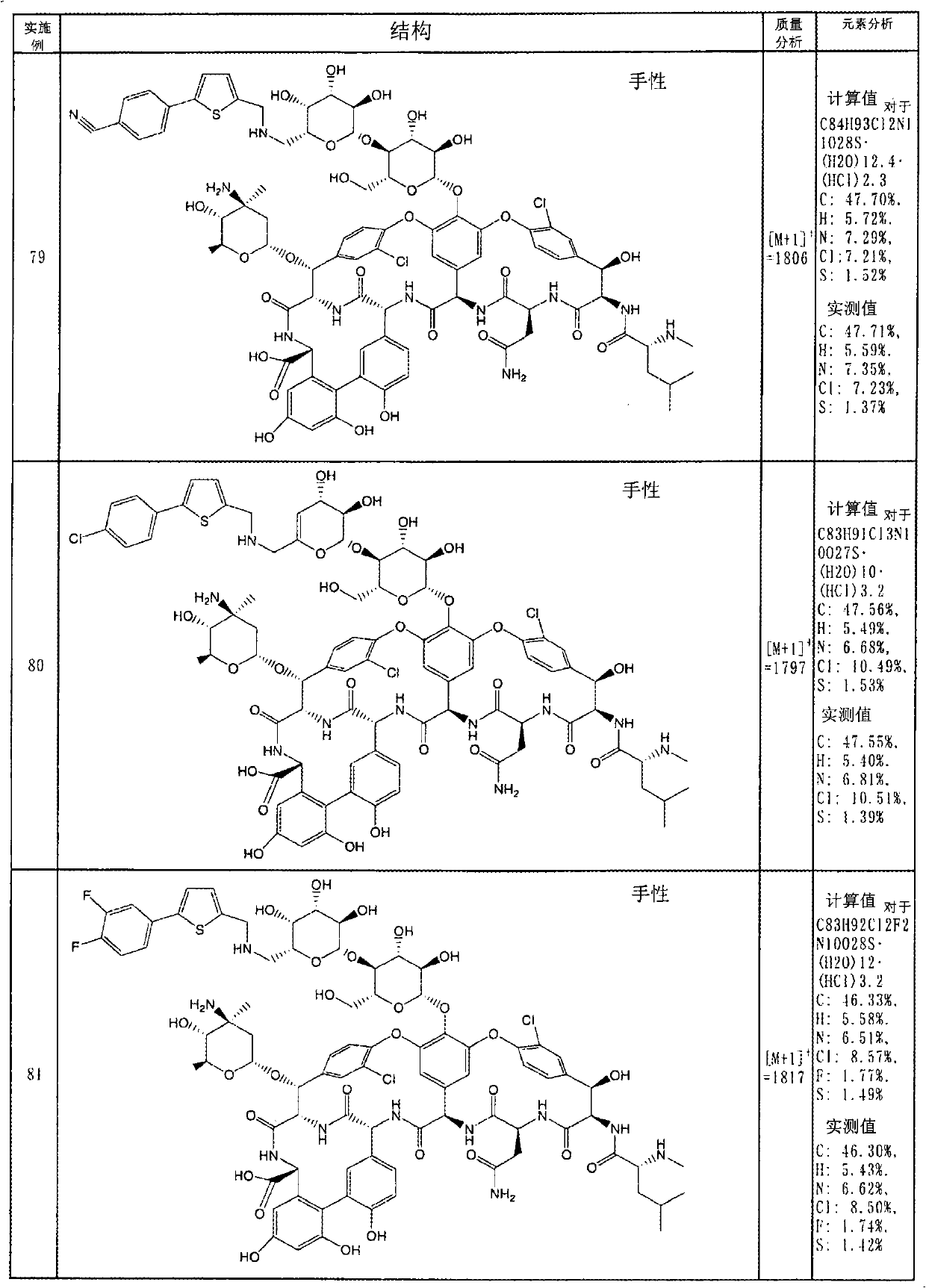
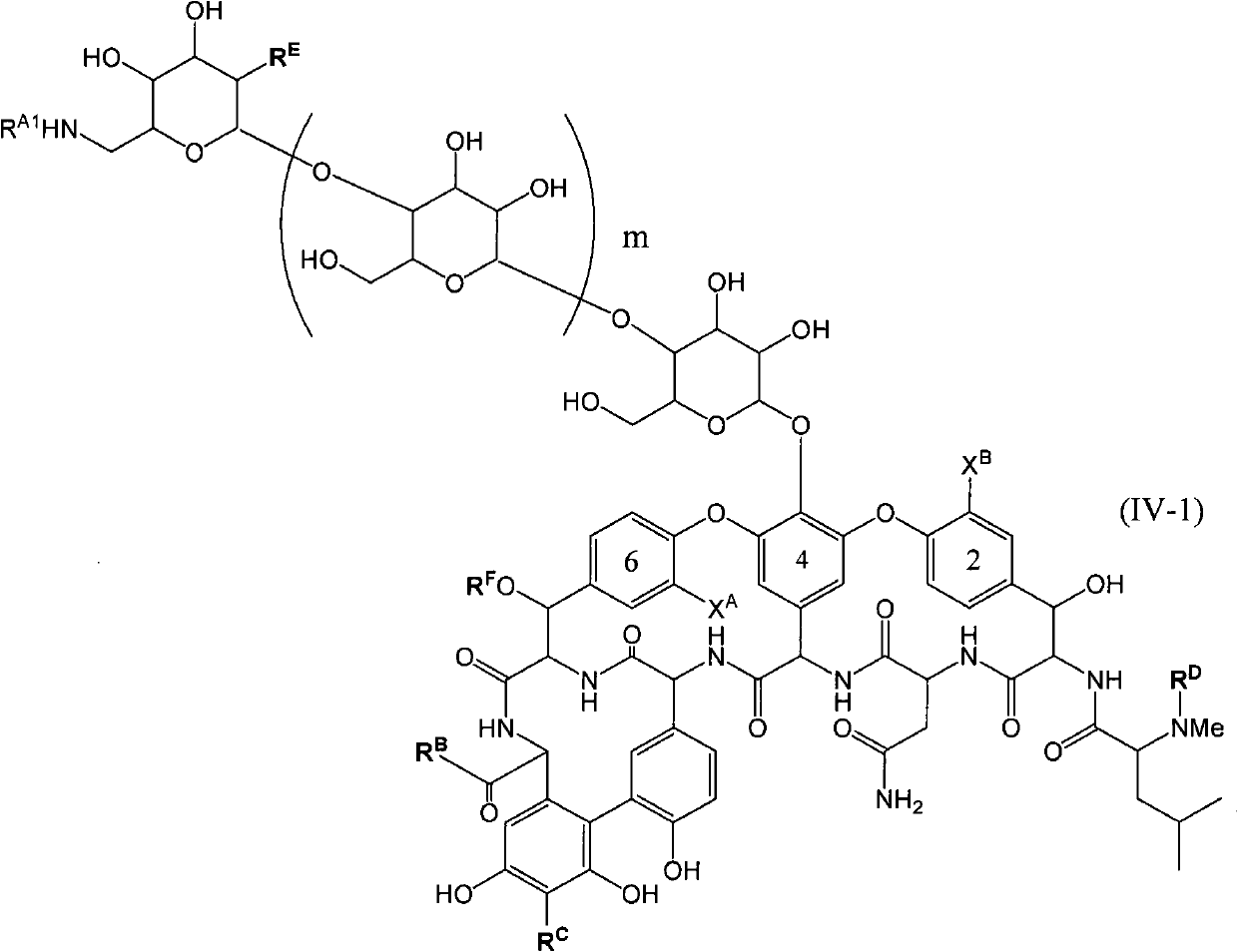
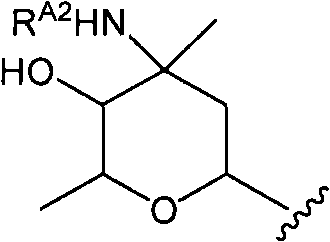
Disclosed is a novel glycopeptide antibiotic derivative. The glycopeptide antibiotic derivative is characterized by having a sugar residue (I) represented by formula (I) [wherein n represents an integer of 1 to 5; Sug's independently represent a monosaccharide, and (Sug)n represents a bivalent sugar residue formed by binding 1 to 5 monosaccharides which are the same as or different from each other; RA1 represents a lower alkyl which may be substituted, a lower alkenyl which may be substituted, or a cycloalkyl which may be substituted; and RE represents OH or NHAc (wherein Ac represents an acetyl)] bound to an aromatic ring in the 4th amino acid residue located in a glycopeptide skeleton. The derivative has an antibacterial activity against a vancomycin-resistant bacterium.
Description
technical field The invention relates to glycosylated derivatives of glycopeptide antibiotics, a preparation method and intermediates thereof. Background technique Glycopeptide antibiotics are antibiotics with complex polycyclic peptide structures produced by various microorganisms and provide antibacterial drugs effective against most Gram-positive bacteria. In recent years, bacteria resistant to penicillins, cephalosporins, etc. have emerged, and infections caused by multidrug-resistant bacteria and methicillin-resistant Staphylococcus (MRSA) have caused serious problems in the medical field. Typically, glycopeptide antibiotics such as vancomycin are effective against such microorganisms, and vancomycin becomes a drug of last resort for infections caused by MRSA and other resistant bacteria. However, specific microorganisms such as vancomycin-resistant enterococci (VRE) are beginning to develop resistance to vancomycin. In addition, Staphylococcus aureus (VRSA) which has...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07K9/00C07G11/00A61K38/00A61P9/00A61P31/04
CPCC07K9/008A61K38/00A61P11/00A61P17/00A61P19/02A61P31/04A61P9/00
Inventor 松井耕平皆川和之吉田修森元健次绪方雄贵
Owner SHIONOGI & CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com