Method for synthesizing azacitidine
A synthesis method and azacitidine technology are applied in the field of azacitidine synthesis, which can solve the problems of affecting the purity of azacitidine finished products, destruction of azacitidine, and general low efficiency, and shorten the silanization reaction time. , low price, and the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] According to the present invention, the synthetic method of described azacitidine comprises the following steps:
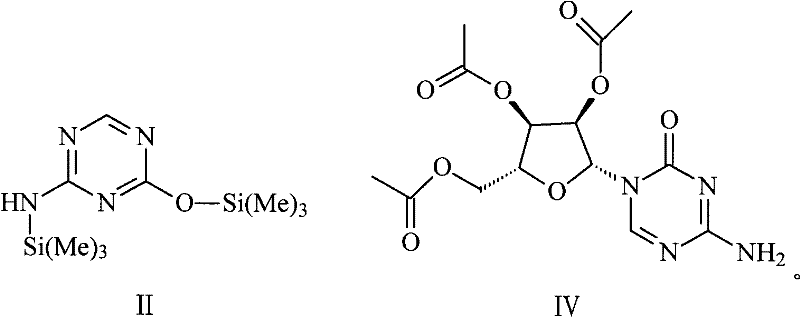
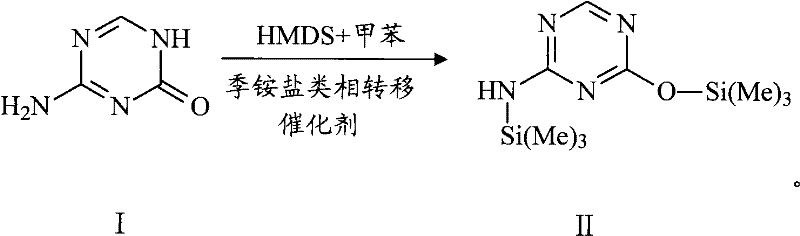
[0031] Step 1. Under the condition of cutting off moisture, bistrimethyldisilamine and 5-azacytosine use toluene as a solvent to carry out a silanization reaction under the catalysis of a quaternary ammonium salt phase transfer catalyst to generate a compound with the structure of formula II , the molar ratio of the 5-azacytosine to the quaternary ammonium salt phase transfer catalyst is 1:0.005-0.5;
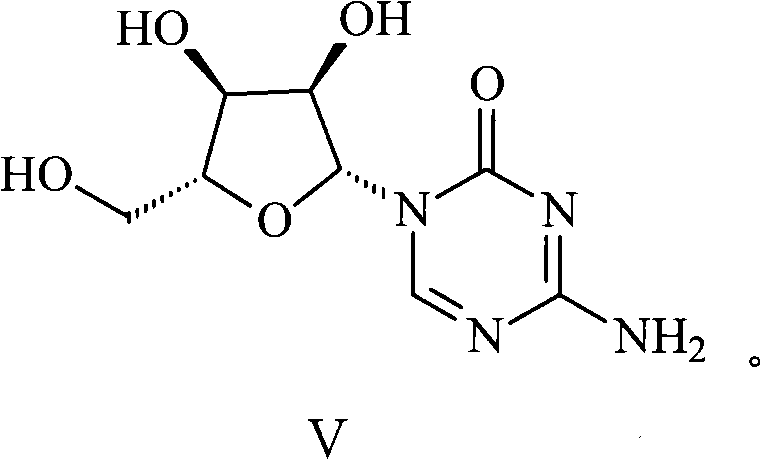
[0032] Step 2, the compound of formula II undergoes a condensation reaction with tetraacetyl ribose to generate a compound with the structure of formula IV, and the compound of formula IV undergoes alcoholysis in an alkaline environment to generate azacitidine;
[0033]
[0034] Wherein, as preferably, the quaternary ammonium salt phase transfer catalyst is tetrabutylammonium bisulfate, tetrabutylammonium bromide, tetrabutylammonium chloride, tetrabutylamm...
Embodiment 1
[0043] Embodiment 1: the synthetic method of azacitidine described in the present invention
[0044] Under nitrogen protection, put 25.8g (0.23mol) of 5-azacytosine and 1.0g (0.003mol) of tetrabutylammonium bisulfate into a 1L three-necked flask, add 125ml of HMDS and 500ml of toluene and stir to raise the temperature to 125°C. The solution was clarified in about 6 hours, and then the solvent was distilled off under reduced pressure to constant weight to obtain N-trimethylsilyl-4-trimethylsilyloxy-2-amine-1,3,5-triazine, namely formula II Compound 65.0 g (0.25 mol).
[0045] Under the protection of nitrogen, after dissolving 65.0g (0.25mol) of the compound of formula II in 500ml of tetrahydrofuran, put it into a 1L three-necked flask and stir, then add 120.0g (0.38mol) of tetraacetyl ribose, then rinse the addition funnel with 100ml of tetrahydrofuran, and Slowly add 64.5ml (0.36mol) of trifluoromethyltrimethylsilicone, and stir the reaction. After 4 hours of reaction, the re...
Embodiment 2
[0047] Embodiment 2: the synthetic method of azacitidine described in the present invention
[0048] Under nitrogen protection, put 25.8g (0.23mol) of 5-azacytosine and 0.4g (0.001mol) of tetrabutylammonium bromide into a 1L three-necked flask, add 120ml (0.575mol) of HMDS and 258ml of toluene and stir to raise the temperature After reaching 125°C, the solution was clarified for about 5 hours, and then the solvent was distilled off under reduced pressure to constant weight to obtain N-trimethylsilyl-4-trimethylsilyloxy-2-amine-1,3,5-triazine , namely 62.0 g (0.24 mol) of the compound of formula II.
[0049] Under the protection of nitrogen, after dissolving 62.0g (0.24mol) of the compound of formula II in 500ml of tetrahydrofuran, put it into a 1L three-necked flask and stir, then add 115.0g (0.36mol) of tetraacetylribose, then rinse the addition funnel with 100ml of tetrahydrofuran, and Slowly add 64.5ml (0.36mol) of trifluoromethyltrimethylsilicone, and stir the reaction. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com