Method for preparing 2,3,3,3-tetrafluoropropylene
A technology of tetrafluoropropene and trifluoropropene, which is used in the preparation of dehydrohalogenation, organic chemistry, etc., can solve the problems of high potential value of the greenhouse effect, warming, and long residence life, and achieves simple equipment requirements and fewer side reactions. , produce easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, a kind of preparation method of 2,3,3,3-tetrafluoropropene:
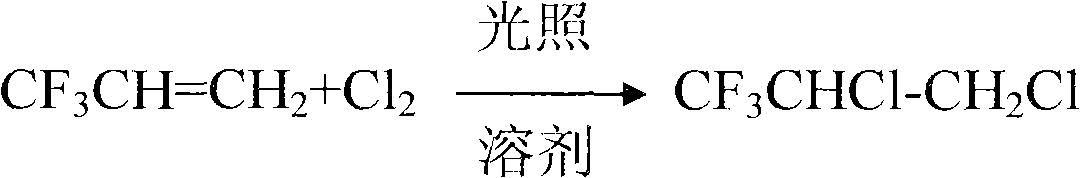
[0023] (a) Add CCl to a 5L glass three-neck flask 4 1kg, under ultraviolet light irradiation, with trifluoropropene 500ml / min, CL 2 420ml / min into the three-necked flask CCl 4 layer, the reaction temperature is 50°C, and the product HFC-243db (CF 3 CHClCH 2 Cl) 5kg.
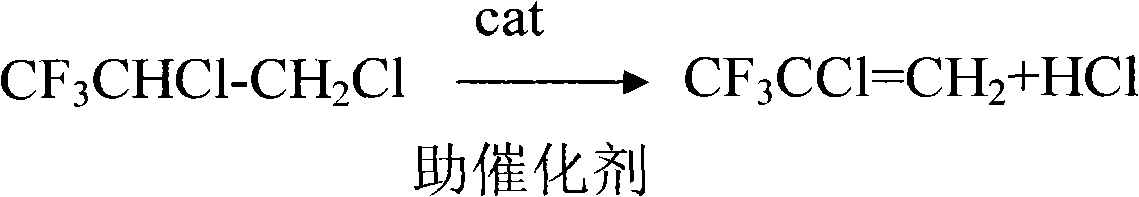
[0024] (b) 3L of industrial liquid caustic soda and 2g of diethylene glycol were added to a 5L glass three-neck flask, the reaction temperature was 60°C, 3kg of HFC-243db was added dropwise in 8 hours, 2.2kg of the product was collected with ice water, and the trifluoroethylene was analyzed by GC Allyl chloride (HCFC-1234xf) content is 92%.
[0025] (c) In a 5L fluorination reaction kettle, add dimethyl sulfoxide 300ml, TiCl 4 20g, 10g of fluorosulfonic acid, 2kg of anhydrous hydrogen fluoride, 1.3kg of chlorotrifluoropropene (HCFC-1234xf), collected in ice water after 18 hours of reaction to obtain 1.43kg of tetrafluorochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com