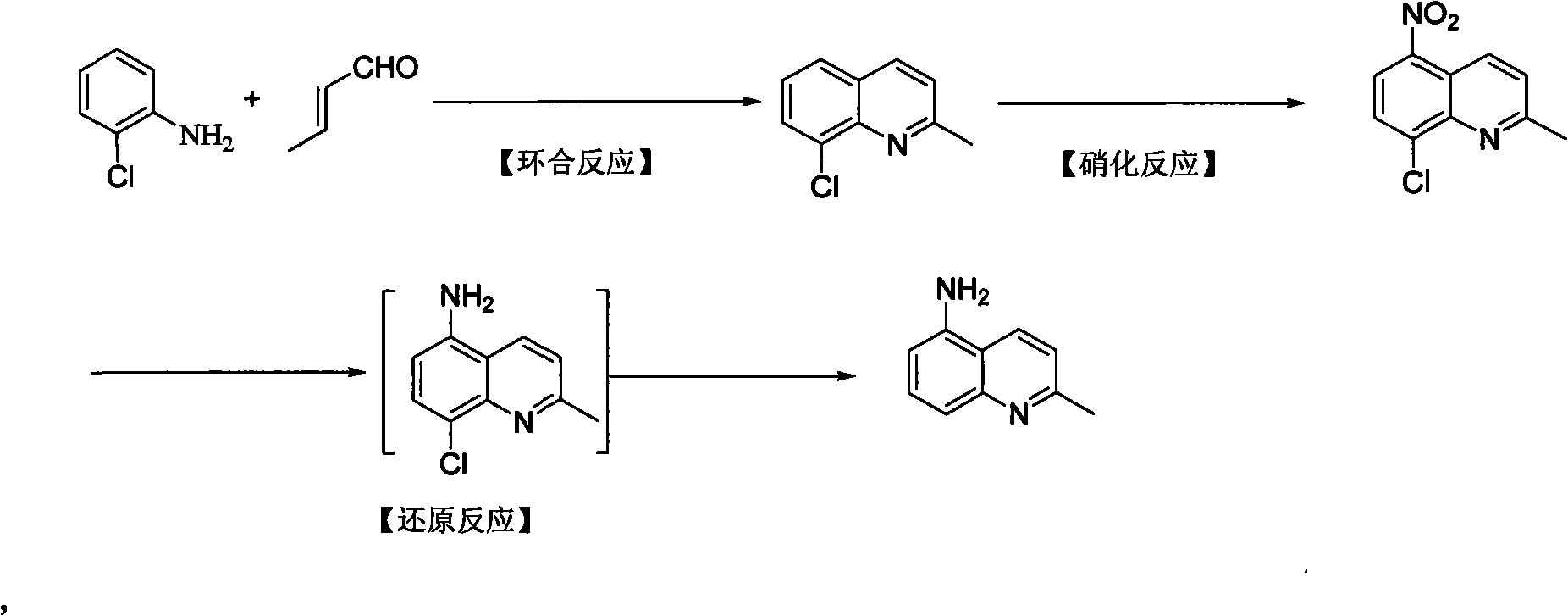
Preparation method of 5-amino-2-methyl quinoline
A technology of methylquinoline and amino group, applied in the field of synthesis of 5-amino-2-methylquinoline, can solve the problems of difficulty, high production cost, low high-temperature cyclization yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 50g of o-chloroaniline, 130.5g of n-butanol, 120g of concentrated hydrochloric acid, 96.3g of chlorobenzoquinone into a 1L reaction bottle, stir mechanically, and heat the oil bath to 90-95°C; mix 33g of crotonaldehyde and 33g of n-butanol evenly , Added dropwise to the reaction flask, heated to reflux for 1h after dropping. Cool down to 80°C, add 53.4g zinc chloride solid in batches, then add 240g isopropyl ether, and reflux for 1h. Sampling analysis, the reaction is complete. Cool to 0°C, keep stirring for 1 h, filter, and collect 83.1 g of solid product. HPLC detection purity ≥ 99%, yield 99% (based on o-chloroaniline).
Embodiment 2
[0028] According to Example 1, 240 g of tetrahydrofuran was used instead of isopropyl ether as a solvent, and 83.4 g of 8-chloro-2-methylquinoline hydrochloride was obtained under the same operation. The purity by HPLC was 98.8%, and the yield was 99.3%.
Embodiment 3
[0030] According to Example 1, the consumption of chloranil was reduced by half, and 48.2 g was cast instead. Other conditions remained unchanged, and finally the product 8-chloro-2-methylquinoline hydrochloride was 53g, the purity was 95%, and the yield was 60%.
[0031] The second step: the preparation of 8-chloro-5-nitro-2-methylquinoline
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com