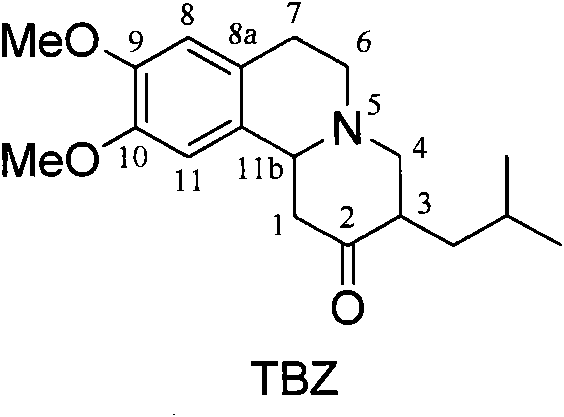
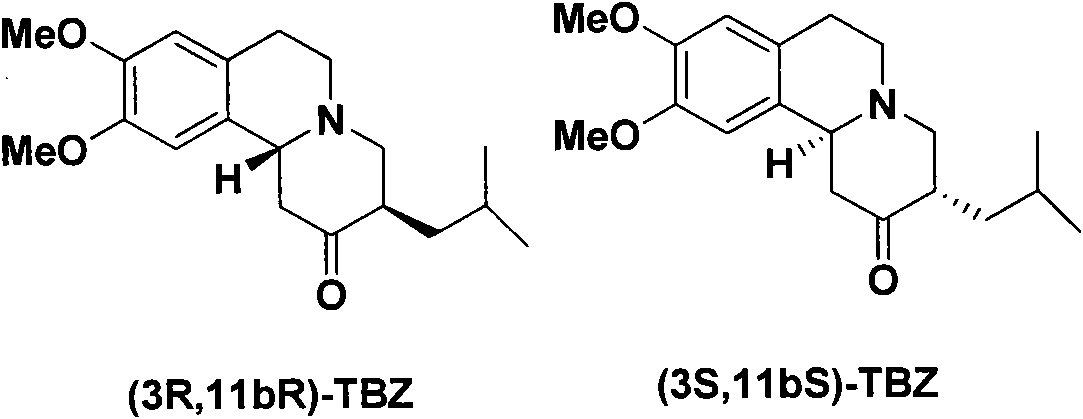
Resolution method of tetrabenazine
A technology of tetrabenazine and levotetrabenazine, applied in the field of chemistry, can solve the problems that optically active tetrabenazine is not suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve 0.3g (0.95mmol) of tetrabenazine and 0.2g (0.95mmol) of (+)-camphorsulfonic acid in an appropriate amount of acetone, reflux for 30 minutes, cool and crystallize, and filter to obtain white crystals of (3R, 11bR)-butylene Benazine.(+)-Camphorsulfonate (0.19 g). The ee value of (3R,11bR)-tetrabenazine free base obtained after dissociation of the salt was 47.8%.
Embodiment 2
[0034] Dissolve 0.3g (0.95mmol) of tetrabenazine and 0.11g (0.48mmol) of (+)-camphorsulfonic acid in an appropriate amount of acetone, reflux for 30 minutes, cool and crystallize, and filter to obtain white crystals of (3R, 11bR)-butylene Benazine (+)-Camphorsulfonate (0.10g), [α]D 25 : +33.2 (MeOH); 1 H NMR (300MHz, MeOD) δ: 6.85(s, 1H), 6.82(s, 1H), 4.64-4.39(m, 1H), 4.06-3.68(m, 7H), 3.53-3.30(m, 3H), 3.23-2.99(m, 3H), 2.73-2.57(m, 2H), 2.34(t, 1H, J=9.3Hz), 2.28(t, 1H, J=9.4Hz), 2.20-1.49(m, 9H) , 1.22-1.16 (m, 1H), 1.09 (s, 3H), 0.98-0.91 (m, 6H), 0.82 (s, 3H). The (3R, 11bR)-butylbenzene obtained after the dissociation of the salt The ee value of nazine free base is 98.4%.
Embodiment 3
[0036] Dissolve 0.8g (2.52mmol) of tetrabenazine and 0.3g (1.29mmol) of (-)-camphorsulfonic acid in an appropriate amount of acetone, reflux for 30 minutes, cool and crystallize, and filter to obtain white crystals of (3S, 11bS)-butylene Benazine (-)-camsylate (0.22g), [α] D 25 : -36.4 (MeOH). 1 H NMR (300MHz, MeOD) δ: 6.86(s, 1H), 6.83(s, 1H), 4.51-4.38(m, 1H), 3.84-3.68(m, 7H), 3.50-3.07(m, 6H), 2.75-2.58(m, 2H), 2.35-2.28(m, 1H), 2.21-1.51(m, 7H), 1.41-1.30(m, 1H), 1.10(s, 3H), 0.99-0.92(m, 6H) ), 0.83(s, 3H). The ee value of the (3S, 11bS)-tetrabenazine free base obtained after the dissociation of the salt was 97.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com