Method for splitting ketoprofen enantiomer by temperature gradient simulated moving bed chromatogram
A technology for simulating moving bed and temperature gradient, applied in organic chemistry methods, chemical instruments and methods, separation of optically active compounds, etc., can solve the problem of not preparing L- and D-ketoprofen enantiomer crystals, complete separation and narrow operating range , affecting SMB separation efficiency and other issues, to achieve the effect of simple and easy operation, high purity and recovery rate, and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
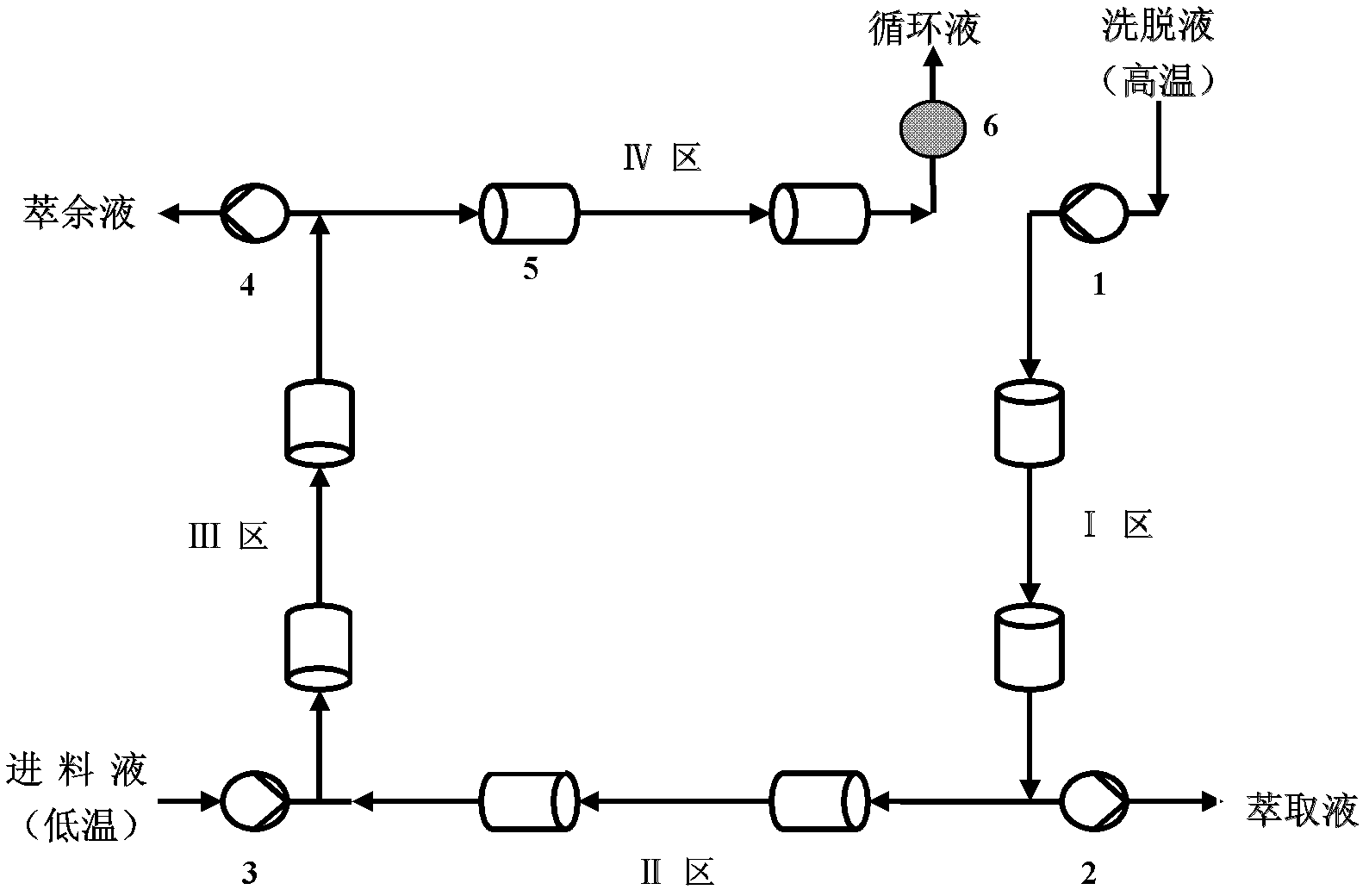
[0035] 1. Preprocessing
[0036] Using a Chiralpak AD chromatographic column, mix chromatographically pure n-hexane, ethanol, and trifluoroacetic acid in a volume ratio of 80: 20: 0.01 to form a mobile phase, and filter it with a 0.22 μm solvent filter membrane. Ultrasonic treatment for 20min was used as the eluent; then the mobile phase was used as the solvent to accurately prepare a ketoprofen racemate solution with a total concentration of 1g / L, which was used as the feed solution.
[0037] 2. Simulated moving bed (SMB) separation
[0038] The operating conditions are as follows:
[0039] Mobile phase: normal hexane: ethanol: trifluoroacetic acid=80: 20: 0.01
[0040] Injection concentration: C F =1.0g / L;
[0041] Number of chromatographic columns: 8 ( , 2 / 2 / 2 / 2 distribution);
[0042] Eluent flow rate: Q D =10.0mL / min;
[0043] Extraction flow: Q E =2.9 mL / min;
[0044] Feed liquid flow rate: Q F =0.4mL / min;
[0045] Raffinate flow rate: Q R =2.1 mL / min; ...
Embodiment 2
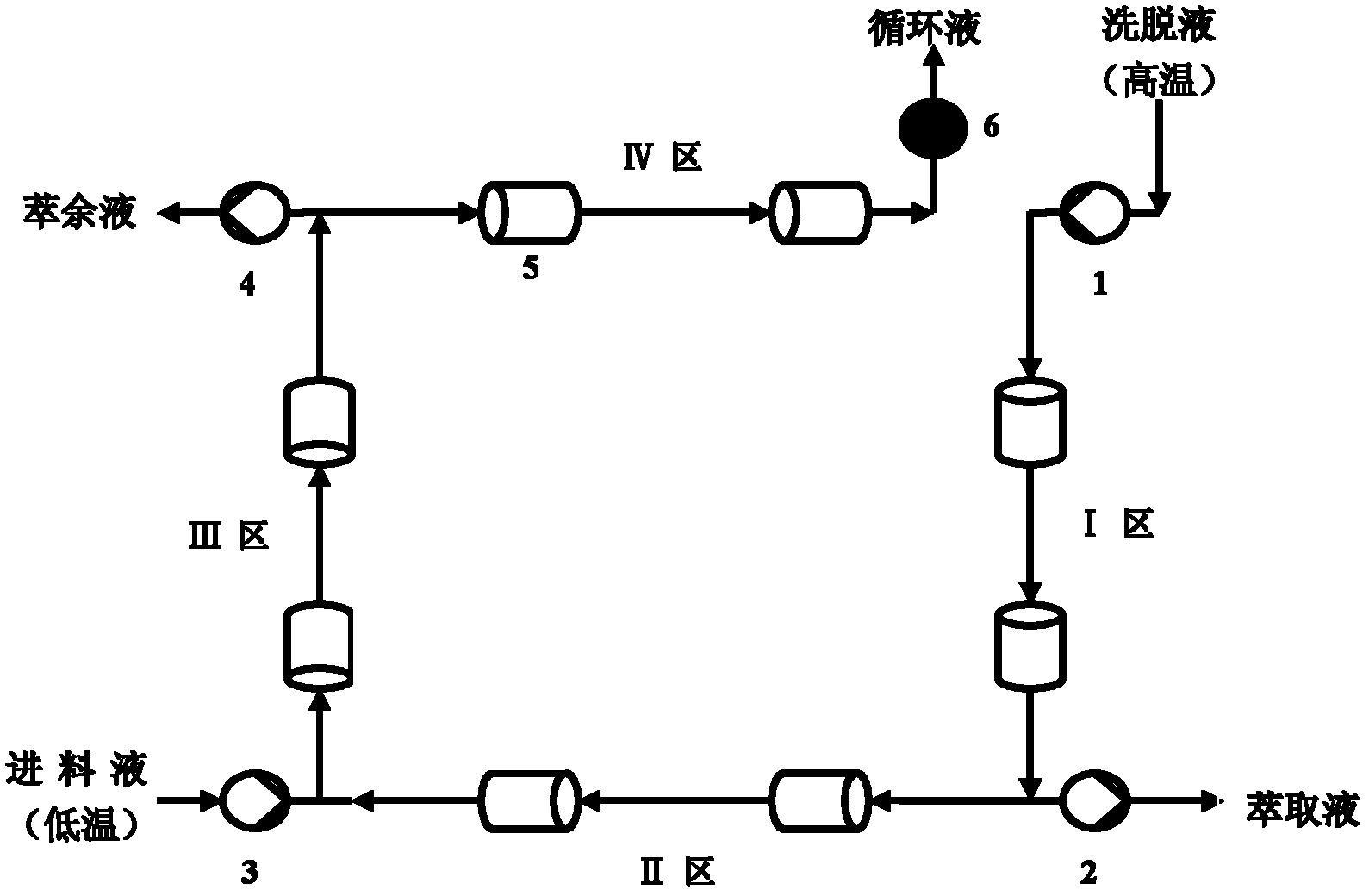
[0077] 1. Preprocessing
[0078] Using a Chiralpak AD chromatographic column, mix chromatographically pure n-hexane, ethanol, and trifluoroacetic acid in a volume ratio of 100: 0: 0.03 to form a mobile phase, and filter it with a 0.22 μm solvent filter membrane. Ultrasonic treatment for 20min was used as an eluent; then the mobile phase was used as a solvent to accurately prepare a ketoprofen racemate solution with a total concentration of 40g / L, which was used as a feed solution.
[0079] 2. Simulated moving bed (SMB) separation
[0080] The operating conditions are as follows:
[0081] Mobile phase composition: normal hexane: ethanol: trifluoroacetic acid=100: 0: 0.03;
[0082] Injection concentration: C F =40.0g / L;
[0083] Number of chromatographic columns: 8 ( , 2 / 2 / 2 / 2 distribution);
[0084] Eluent flow rate: Q D =20.0 mL / min;
[0085] Extraction flow: Q E =9.2 mL / min;
[0086] Feed liquid flow rate: Q F =0.4 mL / min;
[0087] Raffinate flow rate: Q R =2.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com