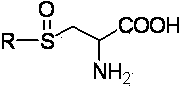
Method for preparing thioalkyl/alkenyl cysteine sulfoxide by fractional crystallization
A cysteine sulfoxide, fractional crystallization technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as by-products and impurities, affecting product purity, toxicity, etc., to avoid Effect of complex process, high product purity and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
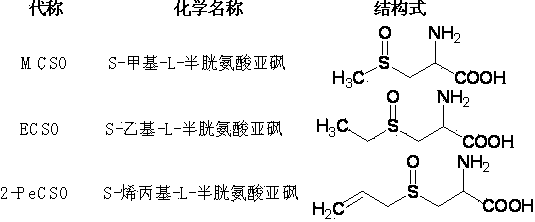
[0045] Example 1: Preparation of (+)S-methyl-L-cysteine sulfoxide ((+)MCSO)
[0046] (1) Take L-cysteine hydrochloride (1mol) and evenly disperse in 3L absolute ethanol, add 3.5mol sodium hydroxide solution (20mol / L) dropwise under magnetic stirring at room temperature, and continue stirring for 5min ; Then add 1.1mol methyl bromide and keep the temperature constant for 6 hours to generate a crude solution of deoxythiomethylcysteine sulfoxide (MCS). The solution was transferred to a clean container, and the pH was adjusted to 5.2 with acid at 30°C. White deoxygenated MCS crystals were formed at 4°C for 12h.
[0047] (2) Filter the MCS crystals obtained in step (1), dry them at 40°C, redissolve them in 10 mL of distilled water containing 1% glacial acetic acid, and heat to boiling. Pour the solution into 150 mL of boiling absolute ethanol for recrystallization. At this time, the MCS crystals will stand upside down in the solvent and the solution will become cloudy. The ...
Embodiment 2
[0052] Example 2: Preparation of (+) S-allyl-L-cysteine sulfoxide ((+) 2-PeCSO)
[0053] (1) Take L-cysteine (1mol) and evenly disperse it in 3L absolute ethanol, add 3.5mol sodium hydroxide solution (20mol / L) dropwise under magnetic stirring at room temperature, and continue stirring for 10min; then add 1.1mol of allyl bromide was reacted at room temperature for 6 hours to generate a crude solution of deoxythioallyl cysteine sulfoxide (2-PeCS). The solution was transferred to a clean container, and the pH was adjusted to 5.5 with acid at 30°C. White deoxygenated 2-PeCS crystals were formed at 4°C for 12h.
[0054] (2) Filter the 2-PeCS crystal obtained in step (1), dry it at 50°C, then redissolve it in 10 mL of distilled water containing 1% glacial acetic acid, and heat it to boiling. Pour the solution into 150 mL of boiling ethanol for recrystallization. The solution was placed at 4°C for 12 hours for recrystallization, and the crystals were filtered and dried at 50°...
Embodiment 3
[0058] Example 3: Preparation of (+) S-propyl-L-cysteine sulfoxide ((+) PCSO)
[0059] (1) Take L-cysteine (1mol) and evenly disperse it in 3L absolute ethanol, add 3.5mol sodium hydroxide solution (20mol / L) dropwise under magnetic stirring at room temperature, and continue stirring for 10min; then add 1.1mol of 1-bromopropane was reacted at room temperature for 6h to generate a crude solution of deoxythiopropyl cysteine sulfoxide (PCS). The solution was transferred to a clean container, and the pH was adjusted to 5.18 with acid at 30°C. White deoxidized PCS crystals were formed at 4°C for 12h.
[0060] (2) Filter the PCS crystals obtained in step (1), dry them at 50°C, then redissolve them in 10 mL of distilled water containing 1% glacial acetic acid, and heat to boiling. Pour the solution into 150 mL of boiling ethanol for recrystallization. The solution was placed at 4°C for 12 hours for recrystallization, and the crystals were filtered and dried at 50°C to obtain a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com