Fenticonazole nitrate, preparation of levorotatory form and dextrorotatory form and application of levorotatory form in preparing antifungal medicaments
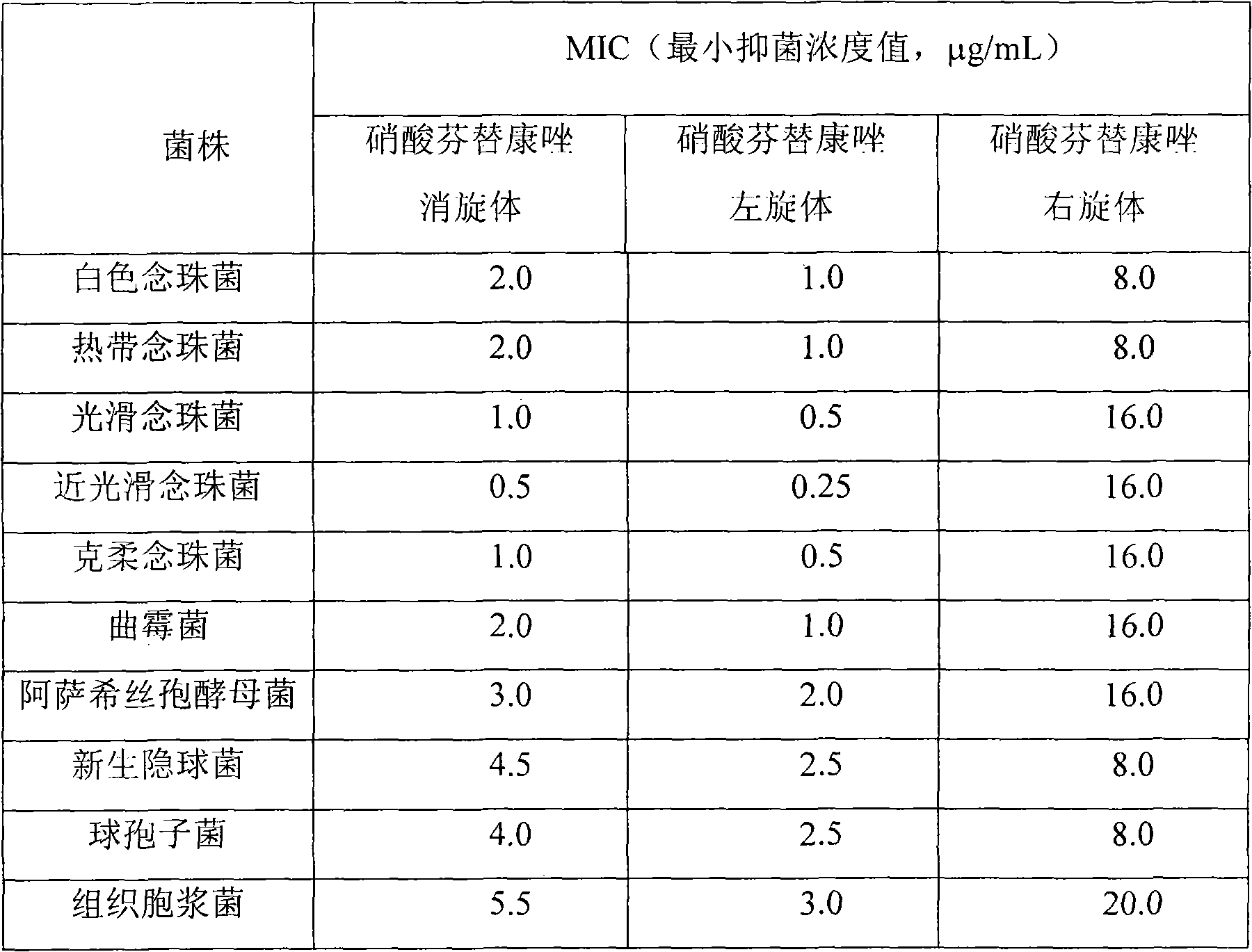
A fenticonazole nitrate and a reaction technology are applied to the preparation of fenticonazole nitrate levorotatory and dextrorotary forms, the antifungal effect of fenticonazole nitrate levorotatory forms, and the application field in the preparation of antifungal drugs, which can solve the problem of Complex operation, affecting industrial application value, cumbersome and other problems, to achieve the effect of high product content, increase industrial application value, and good industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0086] 1 Preparation of Fenticonazole Nitrate
[0087] Step 1, the preparation of copper phenylmercapto
[0088] 28.6g of cuprous oxide, 44g of thiophenol, and 500ml of ethanol were added to the reaction flask, heated to reflux and stirred for 3 hours, and the solvent was recovered under reduced pressure to obtain about 69g of the product. (yield 99%)
[0089] Step 2, the preparation of p-iodobenzoic acid
[0090] A. Add 260mL of 24% sulfuric acid and 13.7g of p-aminobenzoic acid into a 1000mL three-necked bottle, heat up and stir until all p-aminobenzoic acid is dissolved, let the solution cool down to 0-5°C. Slowly add 25 mL of 7.3 g of sodium nitrite solution dropwise, stir for 40 minutes to allow it to fully react, then add 0.6 g of urea, filter to obtain a diazonium salt solution, and store at low temperature.
[0091] B. Dissolve 17.5g of potassium iodide in 160mL of distilled water, raise the temperature at 40°C, keep the temperature constant, slowly add the diazoniu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com