Pharmaceutical composition using herbal extract for prevention and treatment of obesity and metabolic disorders
A technology of metabolic syndrome and composition, which is applied in the field of composition for the prevention or treatment of obesity and metabolic syndrome using compound medicinal materials, which can solve the problems of little effect of preventing hyperlipidemia, high price, and precious materials , to achieve excellent glucose absorption, improve neutral lipids in blood and blood sugar levels, and excellent weight gain inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0051] Preparation Example: Preparation of Compound Extract
[0052] (1) 10 times the weight of 70% ethanol aqueous solution was mixed with 1Kg of dried Evodia rutaecarpa, Rhizoma Imperatae Rhizoma Imperatae and Qingpi which were cut into appropriate sizes, heated, and extracted at 85° C. every 6 hours. Afterwards, filter and collect the filtrate separately, and then mix 10 times the weight of 70% ethanol aqueous solution with the filtered medicinal material, heat it, and perform two extractions at 85° C. for 6 hours. Afterwards, it was filtered to obtain the second filtrate, and the first filtrate and the first filtrate were rapidly frozen at minus 70° C. for 12 hours, vacuum-dried at a pressure of 0.23 Torr or lower for more than 36 hours, and then pulverized to obtain each dry powder.
[0053] For the obtained dry powder of extracts of Evodia rutaecarpa, Rhizoma Imperatae, and bark extract, 1 part by weight of root extract of Evodia rutaecarpa and 3 parts by weight of bar...
Embodiment 1
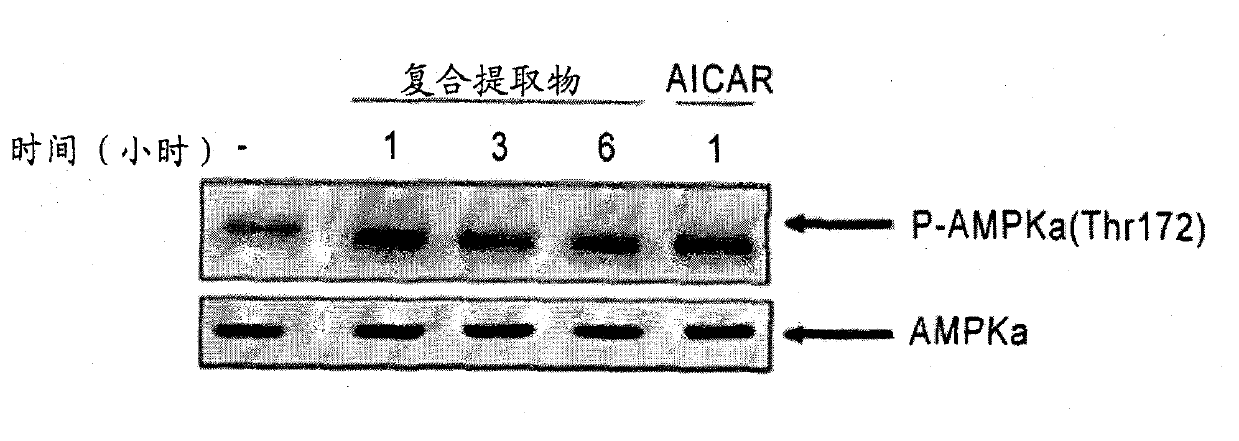
[0056] Example 1: AMPK enzyme activity assay of composite extract
[0057] The level of AMPK activity was investigated over time after the cells were treated with the complex extract of the preparation example. Since the activation of AMPK enzyme increases glucose absorption, inhibits fatty acid synthesis, and induces fatty acid oxidation promotion, this experiment can confirm the possibility of preventing or treating obesity and metabolic syndrome of the composite extract of the present invention.
[0058] figure 1 As confirmed in, the composite extract of the present invention greatly increased AMPK enzyme expression compared to AICAR as a positive control group.
Embodiment 2
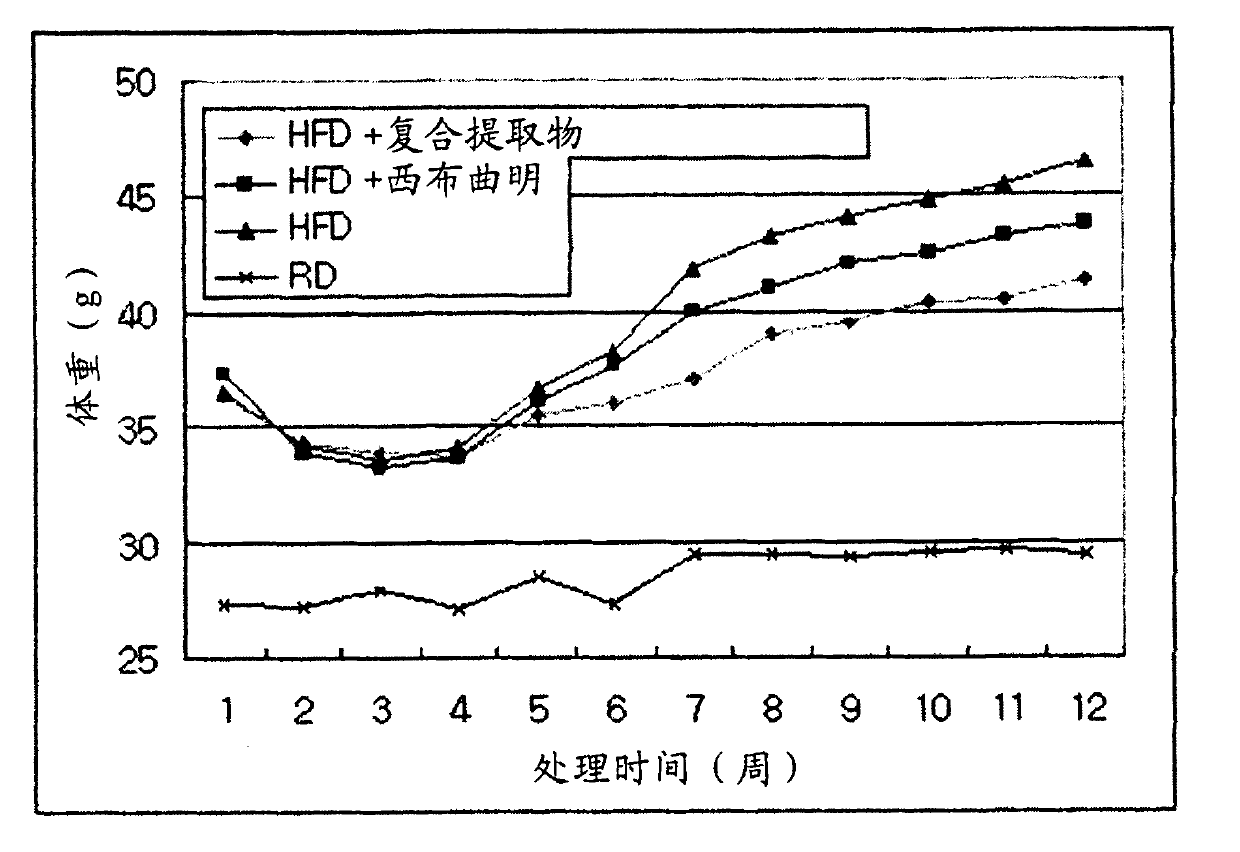
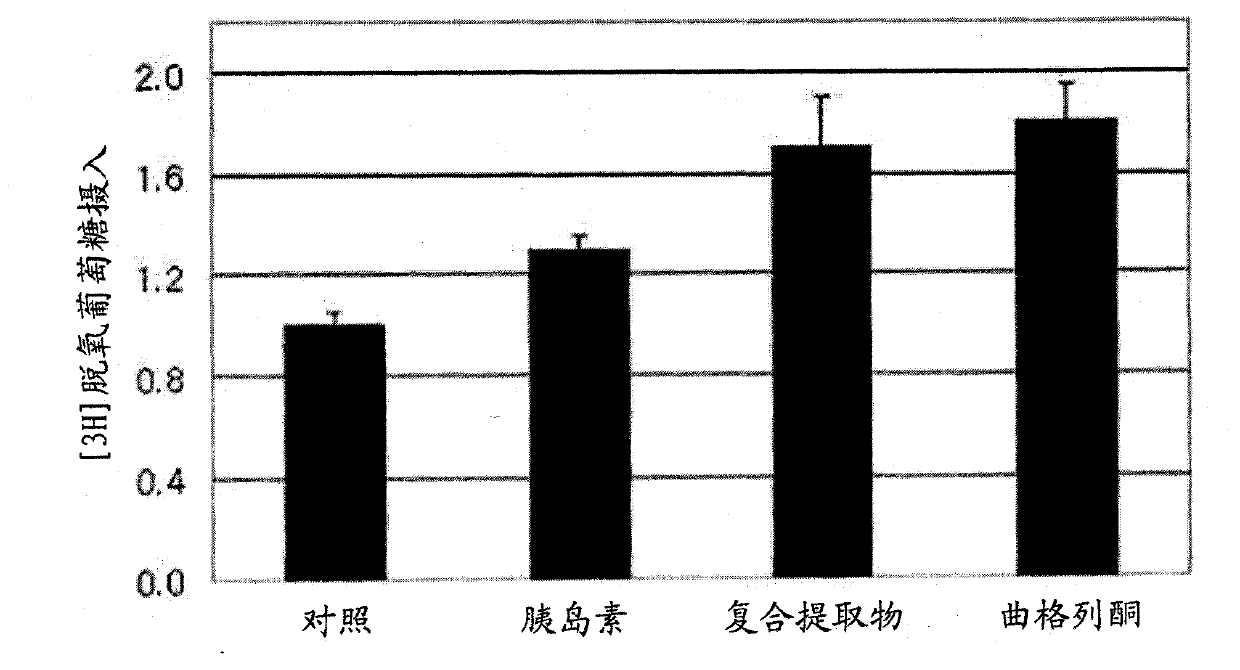
[0059] Example 2: Examination of the effect of compound extracts on weight gain in an animal model and investigation of its effect on fat accumulation and plasma fat and sugar
[0060] (1) The experimental animals were 6-week-old C57BL / 6 mice of 20-25 g purchased from the Central Experimental Animals. They were allowed to ingest food and water freely in the animal room. After 1 week, they were divided into 4 groups as follows, and normal diet (Regular Diet , RD) and 45% high fat diet (High Fat Diet, HFD) for 6 weeks. After 6 weeks, the mice were re-divided according to body weight, and in the case of HFD, 5 mice per cage were housed at an average of 36-37 g, and the experiment was carried out by assigning 2 cages per group (10 mice per group). The weight of a normal meal is an average of 27g. The complex extract of the present invention was orally administered to mice fed a high-fat diet for 10 weeks, and the changes in body weight and intake of the mice during the perio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com