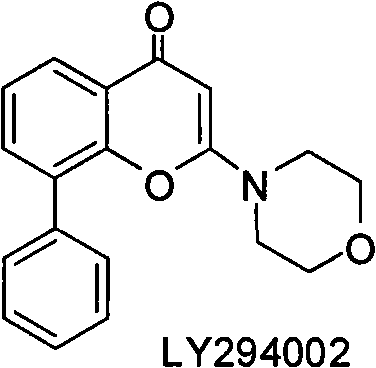
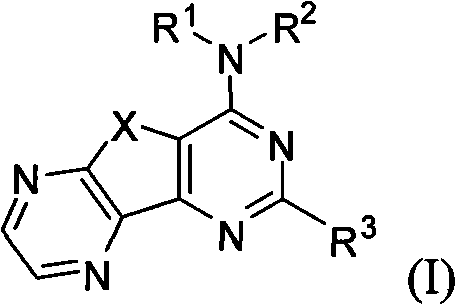
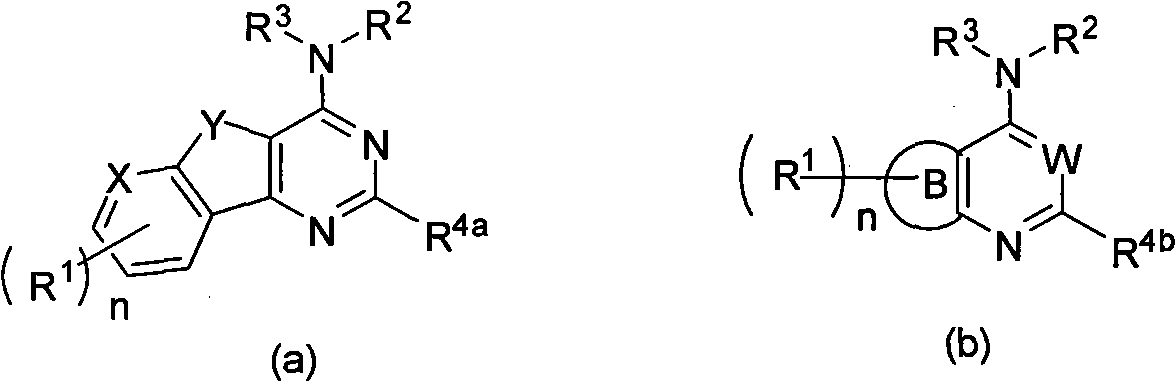
Condensed heteroaryl derivative, and preparation method and application thereof
A kind of derivative and heteroaryl technology, applied in the field of condensed heteroaryl derivatives and preparation thereof, can solve the problems such as no explanation, no disclosure of inhibitory effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] 3-(4-Morpholinopyrazino[3′,2′:4,5]thieno[3,2-d](2-pyrimidinyl))anisole
[0072]
[0073] Starting with 2-chloro-3-cyanopyrazine. 2-Chloro-3-cyanopyrazine is ring-closed with compound (Ia-1) to obtain compound (Ib-1). Compound (Ib-1) is reacted with m-methoxybenzoyl chloride to give compound (Ic-1) as an acylated product. Compound (Ic-1) is reacted with ammonia to give compound (Id-1). Compound (Id-1) undergoes a ring-closing reaction in the presence of a base to obtain compound (Ie-1). (Ie-1) compounds are converted into reactive (If-1) compounds by conventional methods. Compound (If-1) (9.2 mmol) was dissolved in 50 ml of anhydrous tetrahydrofuran, 30 ml of morpholine was added dropwise thereto under an ice-water bath, and heated to reflux for 1.5 hours. Cool down to room temperature, extract repeatedly 3 times with ethyl acetate, combine the organic phases, wash 3 times with saturated brine, and dry over anhydrous sodium sulfate. Silica gel column chromatograp...
Embodiment 2
[0078] 3-(4-Morpholinopyrazino[3′,2′:4,5]thieno[3,2-d](2-pyrimidinyl))phenol
[0079] The compound (1) (6.33 mmol) was dissolved in 100 ml of acetic acid and 120 ml of hydrogen bromide (40% aqueous solution), and heated under reflux overnight. Cool down to room temperature, concentrate under reduced pressure, add saturated aqueous sodium bicarbonate solution to adjust to weak alkalinity, extract with ethyl acetate three times, combine organic phases, and dry over anhydrous sodium sulfate. Column chromatography separated to obtain 0.58 g of a yellow solid (compound 2), with a yield of 25.1%.
[0080] h 1 -NMR(DMSO):
[0081] δ9.59(s, 1H), δ9.02(d, 1H), δ8.91(d, 1H), δ7.99-7.94(m, 2H), δ7.34(t, 1H), δ6.92 (d, 1H), δ4.05(t, 4H), δ3.85(t, 4H)
[0082] ESI (+) m / z: 366
Embodiment 3
[0084] 3-(4-Morpholinopyrazino[3′,2′:4,5]furo[3,2-d](2-pyrimidinyl))phenol
[0085]
[0086] Starting with 2-chloro-3-cyanopyrazine. 2-Chloro-3-cyanopyrazine is ring-closed with compound (Ia-3) to obtain compound (Ib-3). The compound (Ib-3) reacts with m-methoxybenzoyl chloride to obtain the acylated compound (Ic-3). Compound (Ic-3) is reacted with ammonia to give compound (Id-3). Compound (Id-3) undergoes a ring-closing reaction in the presence of a base to obtain compound (Ie-3). (Ie-3) compounds are converted into reactive (If-3) compounds by conventional methods. Compound (If-3) is reacted with morpholine to give compound (Ig-3). Compound (Ig-3) (3.2 mmol) was dissolved in 50 ml of acetic acid and 60 ml of hydrogen bromide (40% aqueous solution), and heated to reflux overnight. Cool down to room temperature, concentrate under reduced pressure, add saturated aqueous sodium bicarbonate solution to adjust to weak alkalinity, extract with ethyl acetate three times, com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com