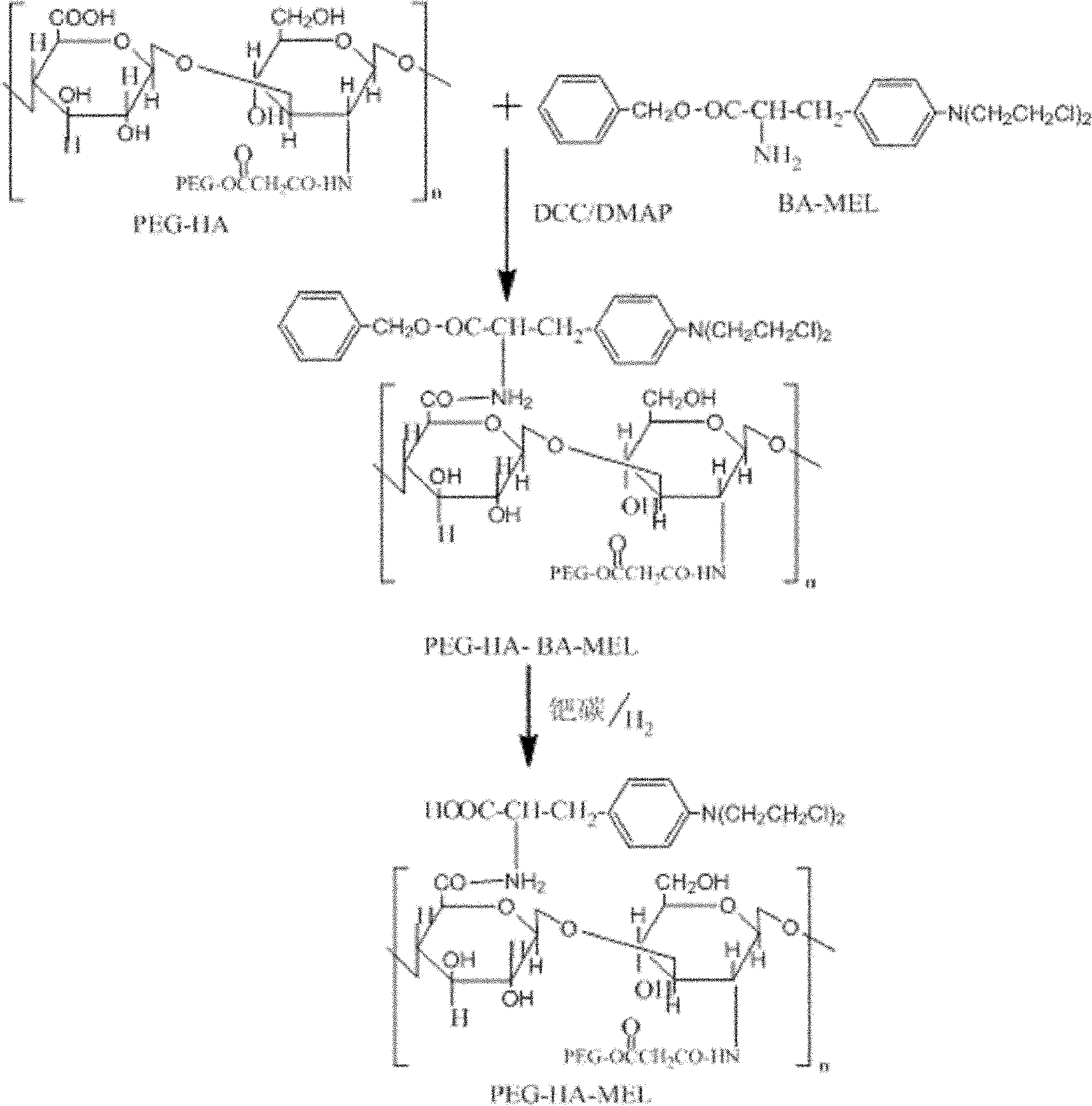
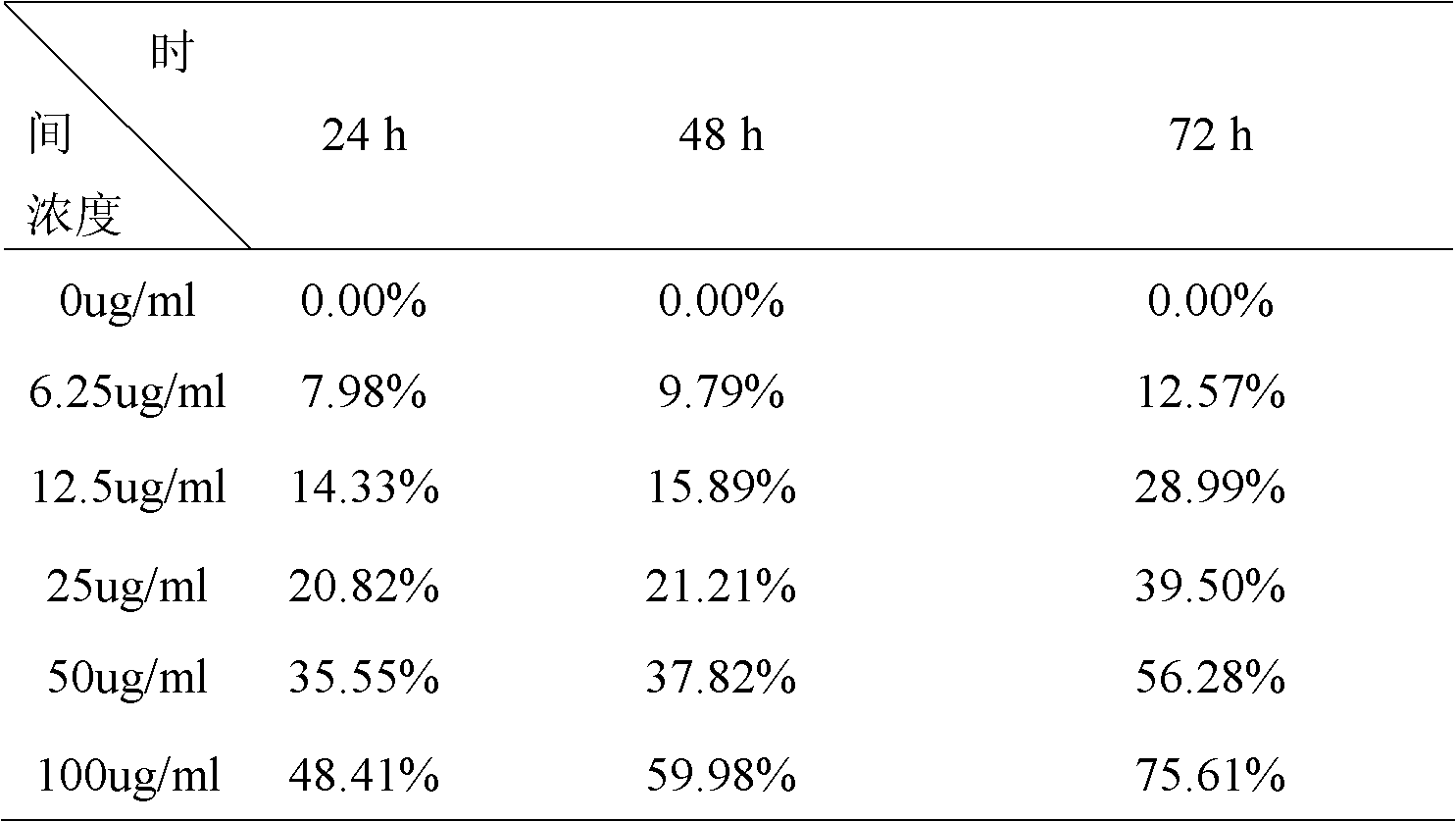
Melphalan multi-targeted drug carrying system, and preparation method and application thereof
A drug-carrying system, the technology of melphalan, applied in the field of medicine, can solve the problems of high toxicity and side effects, poor cell selectivity, etc., achieve wide applicability, expand the scope of application, and improve the effect of drug invisibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Preparation of PEG-HA-MEL Components
[0042] (1) Synthesis and purification of carboxyl-terminated polyethylene glycol
[0043] 10g (5mmol) PEG-2000 was placed in a 250mL three-necked flask with a condenser, a stirrer and a thermometer, and 100mL of chloroform (CaH 2 After the dissolution, add 2.5g (25mmol) succinic anhydride, heat to dissolve, add 2mL of dry pyridine at the same time, reflux reaction under stirring for 6 hours, stop the reaction and cool to room temperature, and the reaction mixture is decompressed Evaporate until it becomes viscous, add a large amount of anhydrous ether to the residue to precipitate a white product, filter it with suction, dissolve the product with 30 mL of dichloromethane, filter off the insoluble matter, add anhydrous ether to precipitate a white product, repeat several times. After suction filtration, vacuum-dry to constant weight to obtain carboxyl-terminated polyethylene glycol, which is weighed;
[0044] (2) Preparation of...
Embodiment 2
[0063] 1. Preparation of PEG-HA-MEL Components
[0064] (1) Synthesis and purification of carboxyl-terminated polyethylene glycol
[0065] Put 20g (5mmol) PEG-2000 in a 500mL three-necked flask with a condenser, a stirrer and a thermometer, add 200mL chloroform (distilled out in the presence of CaH2) to dissolve, and add 5g (50mmol) succinic anhydride after the dissolution is complete, Heat to dissolve, add 4mL of dry pyridine at the same time, reflux reaction under stirring for 6 hours, stop the reaction and cool to room temperature, the reaction mixture is evaporated under reduced pressure until it becomes viscous, the residue is added with a large amount of anhydrous ether to precipitate a white product, and suction filtered , Dissolve the product with 60 mL of dichloromethane, filter off the insoluble matter, add anhydrous ether to precipitate a white product, repeat several times. After suction filtration, vacuum-dry to constant weight to obtain carboxyl-terminated polye...
Embodiment 3
[0087] 1. Preparation of PEG-HA-MEL Components
[0088] (1) Synthesis and purification of carboxyl-terminated polyethylene glycol
[0089] Put 40g (5mmol) PEG-2000 in a 1000mL three-necked flask with a condenser, a stirrer and a thermometer, add 400mL chloroform (steamed out in the presence of CaH2) to dissolve, add 10g (100mmol) succinic anhydride after the dissolution is complete, Heat to dissolve, add 8 mL of dry pyridine at the same time, reflux for 6 hours under stirring, stop the reaction and cool to room temperature, the reaction mixture is evaporated under reduced pressure until it becomes viscous, the residue is added with a large amount of anhydrous ether to precipitate a white product, and suction filtered , Dissolve the product with 120 mL of dichloromethane, filter off the insoluble matter, add anhydrous ether to precipitate a white product, repeat several times. After suction filtration, vacuum-dry to constant weight to obtain carboxyl-terminated polyethylene gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com