Safe method for preparing nitropropane by vapor-phase nitration
A nitropropane and gas phase technology, applied in the preparation of nitro compounds, organic chemistry, etc., can solve the problems of low conversion rate and selectivity of low-carbon alkanes and nitrogen oxides, poor safety, and the selectivity of nitropropane needs to be improved. Achieve the effect of simple structure, easy operation, safe and continuous process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
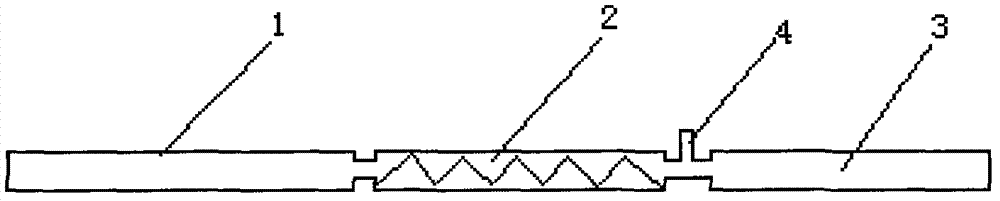
[0022] Such as figure 1 Shown, the present invention adopts single channel reactor to carry out nitration and prepare nitropropane. The inner diameter of the reaction pipe is 4 mm, the length is 1000 mm, the aspect ratio is 250:1, and the specific surface area is 0.82. The volume of the reaction pipe 1: the volume of the mixing pipe 2: the volume of the preheating pipe 3 = 1: 0.6: 0.6. The molar ratio of propane and 65wt% nitric acid is 4:1, the operating pressure is 0.1Mpa, propane enters the preheating pipeline 3 through the flowmeter, the preheating temperature is 350°C, the nitric acid is pumped into the tee port 4 through the metering pump, and the mixing temperature is 250°C , The materials are fully mixed in the mixing pipe 2 and then enter the reaction pipe 1, the reaction temperature is 440°C, the residence time is 0.8s, and the reaction is 1h. The product is condensed by the separator and then separated. The upper layer is the organic phase and the lower layer is the...
Embodiment 2
[0024] The process and the reactor used are the same as in Example 1, except that the molar ratio of propane and nitric acid is 2:1, the operating pressure is 0.5Mpa, and the residence time is 1.4s. The conversion rate of propane is 22.3%, and the selectivity of nitropropane is 81.7% %.
Embodiment 3
[0026] The process and the reactor used are the same as in Example 1, except that the mixing temperature is 200°C, the preheating temperature is 300°C, and the reaction temperature is 435°C. The conversion of propane was 20.8%, and the selectivity to nitropropane was 84.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com