Venlafaxine slow-release preparation and preparation method thereof
A technology of sustained-release preparations and venlafaxine, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, and medical preparations containing active ingredients, etc., which can solve the potential safety hazards of operators, high cost, and high viscosity. problems, to achieve the effect of reducing the number of medications, lowering equipment requirements, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
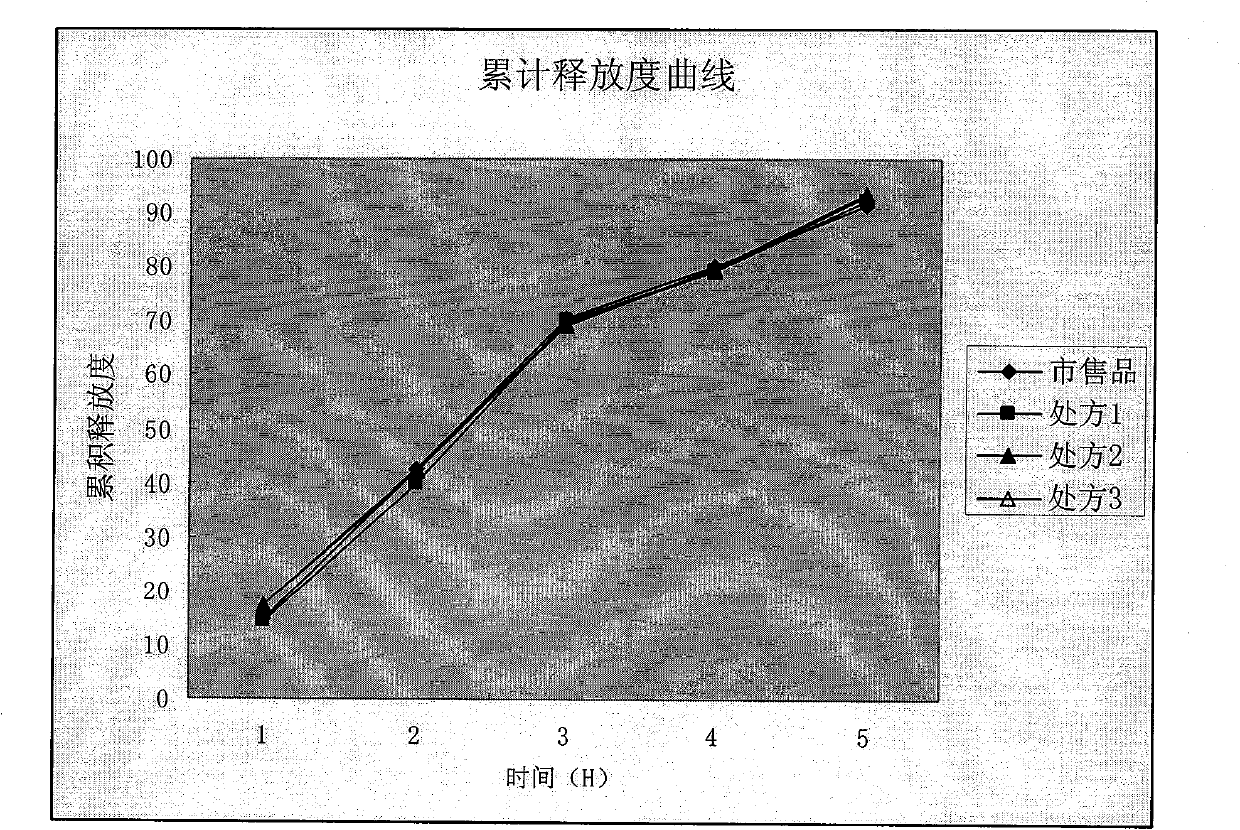
Image
Examples
Embodiment 1
[0020] Composition of pellets:
[0021] 16 parts of microcrystalline cellulose blank pills, 80 parts of venlafaxine, 78 parts of microcrystalline cellulose, 20 parts of dextrin, 12 parts of hypromellose, 14 parts of ethyl cellulose, 210ml of water
[0022] Coating composition:
[0023] 330 parts of polyacrylic resin, 51 parts of talcum powder, 0.4 parts of simethicone
[0024] Preparation:
[0025] (1) Preparation of ball core: sieve and mix the prescribed amount of venlafaxine, microcrystalline cellulose, dextrin, hypromellose, and ethyl cellulose, and put them into the powder supply chamber, and the microcrystalline cellulose is blank The pellets are placed in the coating granulator, and the powder is supplied while spraying water until the powder is all layered on the inert core.
[0026] (2) Coating solution configuration: take the prescribed amount of talcum powder, defoamer and 60ml of water and mix evenly, and then mix evenly with polyacrylic acid resin to obtain a c...
Embodiment 2
[0029] Composition of pellets: 18 parts of microcrystalline cellulose blank pills, 85 parts of venlafaxine, 83 parts of microcrystalline cellulose, 20 parts of dextrin, 12 parts of hypromellose, 13 parts of ethyl cellulose, 220ml of water
[0030] Coating composition: 340 parts of polyacrylic acid resin, 48 parts of talcum powder, 0.3 part of simethicone
[0031] Preparation:
[0032] (1) Preparation of ball core: sieve and mix the prescribed amount of venlafaxine, microcrystalline cellulose, dextrin, hypromellose, and ethyl cellulose, and put them into the powder supply chamber, and the microcrystalline cellulose is blank The pellets are placed in the coating granulator, and the powder is supplied while spraying water until the powder is all layered on the inert core.
[0033] (2) Coating solution configuration: take the prescribed amount of talcum powder, defoamer and 58ml of water and mix evenly, and then mix evenly with polyacrylic acid resin to obtain a coating solution....
Embodiment 3
[0036] Composition of pellets: 11 parts of microcrystalline cellulose blank pills, 62 parts of venlafaxine, 79 parts of microcrystalline cellulose, 28 parts of dextrin, 10 parts of hypromellose, 11 parts of ethyl cellulose, 180ml of water
[0037] Coating composition: 310 parts of polyacrylic acid resin, 43 parts of talcum powder, 0.3 part of simethicone
[0038] Preparation:
[0039] (1) Preparation of ball core: sieve and mix the prescribed amount of venlafaxine, microcrystalline cellulose, dextrin, hypromellose, and ethyl cellulose, and put them into the powder supply chamber, and the microcrystalline cellulose is blank The pellets are placed in the coating granulator, and the powder is supplied while spraying water until the powder is all layered on the inert core.
[0040] (2) Coating solution configuration: take the prescribed amount of talcum powder, defoamer and 48ml of water and mix evenly, and then mix evenly with polyacrylic acid resin to obtain a coating solution. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap