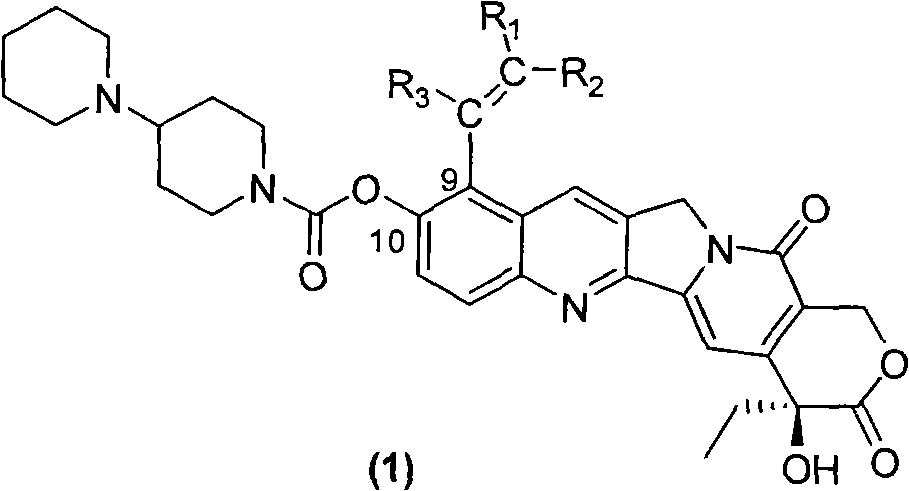
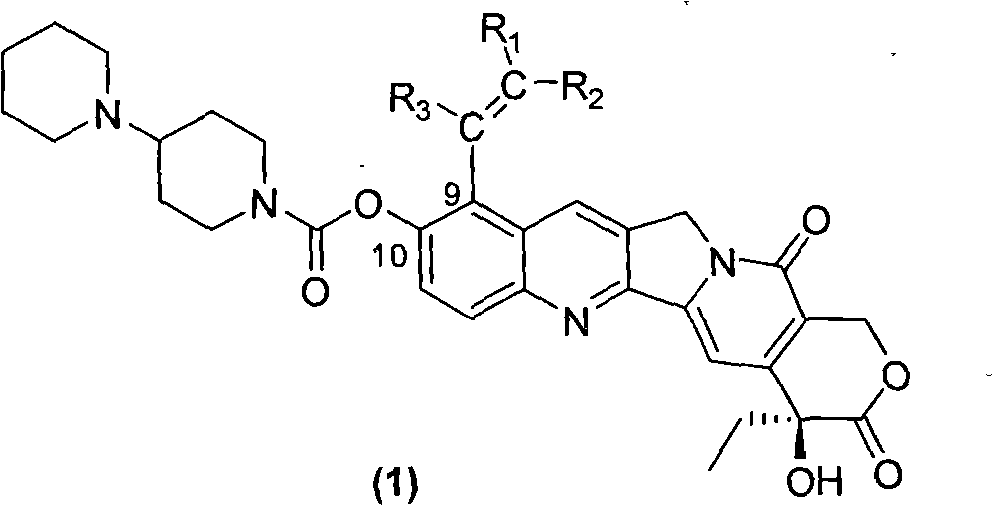
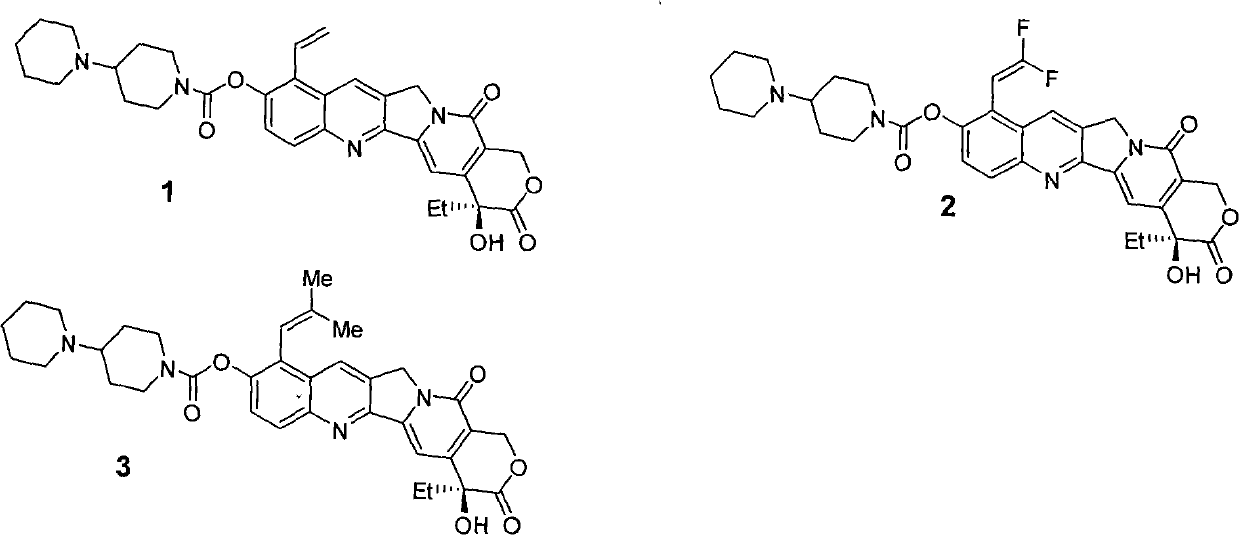
Camptothecin derivative with antitumor activity
A technology of drugs and compounds, applied in the field of camptothecin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The specific preparation method of compound 1:
[0046] Preparation of Intermediate Compound A:
[0047] 10-Hydroxycamptothecin (365mg, 1eq) was dissolved in 1,4-dioxane (5.0ml). To this solution was added N-iodosuccinimide (225mg, 1eq) at 0°C . Stir at room temperature for 3 hours. The final solution was spin-dried under vacuum, and the remaining solid was recrystallized in ethanol to obtain 400 mg of pure compound A (yield 82%).
[0048] Preparation of Intermediate Compound B:
[0049] Compound A (400mg, 1 equivalent) was dissolved in 1,4-dioxane (5.0ml), and chloromethyl methyl ether (0.2ml, 3 equivalents) was added to the solution at 0°C, and N, N' - Diisopropylethylamine (0.4ml, 3eq). Stir at room temperature for 10 hours. The final solution was spin-dried under vacuum, and the final product was separated by silica gel column chromatography to obtain 395 mg of pure compound B (90% yield).
[0050] Preparation of Intermediate Compound C:
[0051] Compound B ...
Embodiment 2
[0056] Preparation of Compound 2-3,
[0057] The preparation method is the same as in Example 1, except that the raw materials used are compounds corresponding to their respective substituents.
Embodiment 3
[0059] Preparation of Tablets Containing Compound 1
[0060]
[0061] Granulated and compressed into 1000 tablets (50mg / tablet).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com