Quality control method for Xuefuzhuyu capsule
A quality control method, Xuefu Zhuyu technology, which is applied in the field of Chinese patent medicines, can solve the problems of not fully reflecting the uniformity and stability of product quality, difficulty in controlling the quality of Xuefu Zhuyu capsules, and large limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Example 1: Take 100g of Chinese medicinal materials peach kernel, 150g of angelica, 100g of red peony root, 100g of citrus aurantii (stir-fried with bran), 75g of Chuanxiong, and 50g of Bupleurum. Add 150g of Achyranthes bidentata, 150g of Rehmannia glutinosa, 75g of bellflower, 50g of licorice and other five flavors and 100g of peach kernel to decoct three times, filter, combine the filtrate, concentrate it into a thick paste with a relative density of 1.15-1.25 (65-70°C), mix it with the above powder Homogenize, make granule, dry, pulverize, sieve, make 1000 capsules, obtain final product; The quality control method of this medicine comprises the following steps:
[0030] (1) Simultaneous determination of amygdalin (C 20 h 27 NO 11 ), paeoniflorin (C 23 h 28 o 11 ), naringin (C 27 h 32 o 14 ) content:
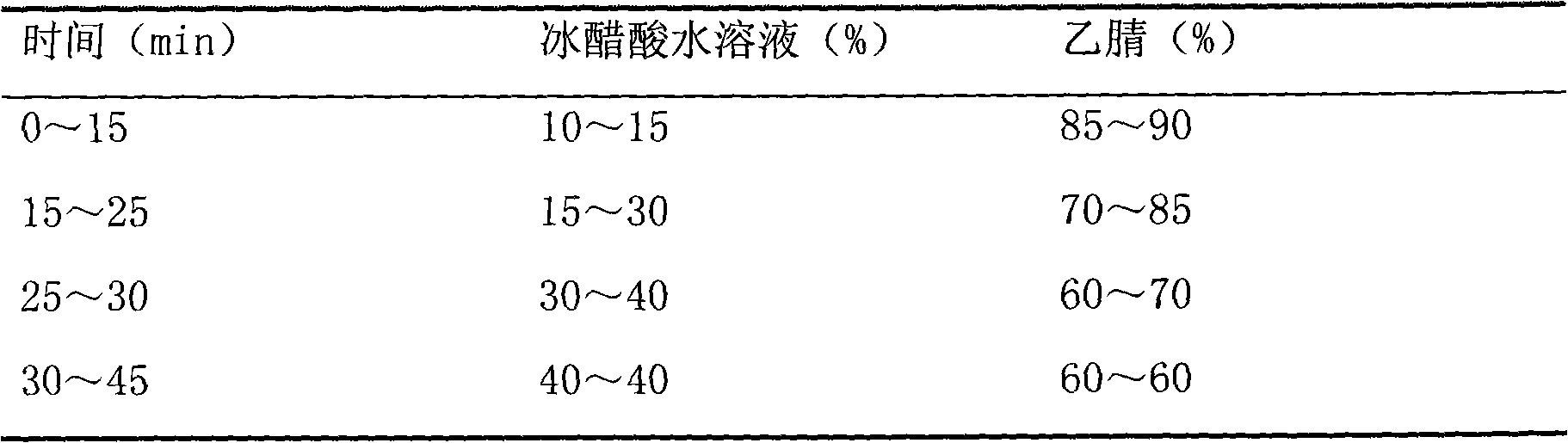
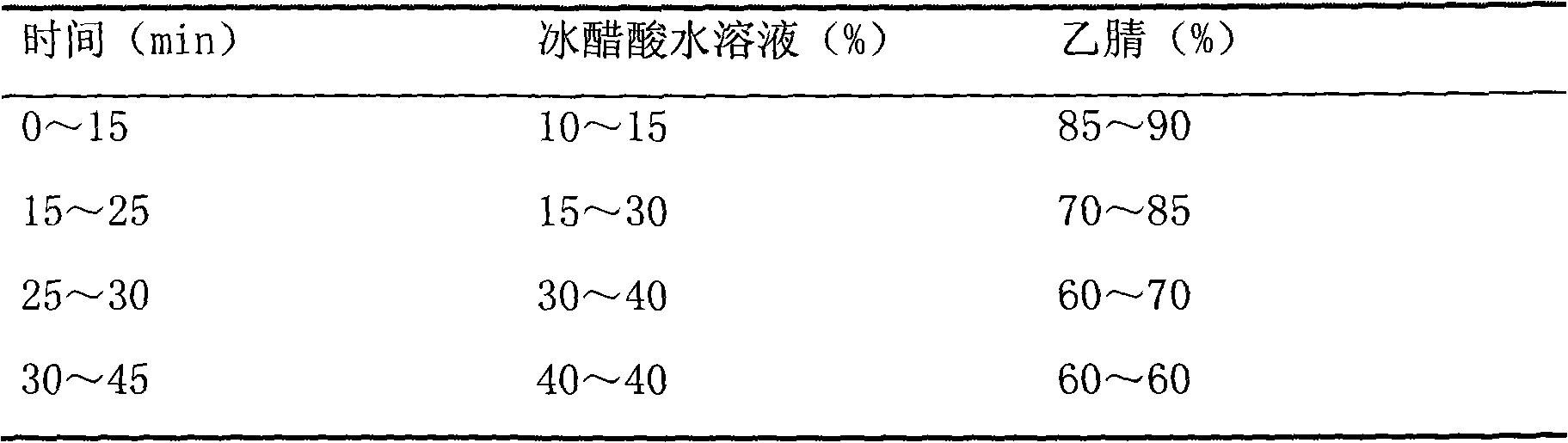
[0031] a. Chromatographic conditions: use octadecylsilane bonded silica gel as filler; acetonitrile-1% glacial acetic acid solution is mobile phase gradient elu...
example 2
[0043] Example 2: Take 100g of peach kernels, 150g of angelica, 100g of red peony root, 100g of citrus aurantii (stir-fried with bran), 75g of Chuanxiong, and 50g of Bupleurum chinensis. Add 150g of Achyranthes bidentata, 150g of Rehmannia glutinosa, 75g of bellflower, 50g of licorice and other five flavors and 100g of peach kernel to decoct three times, filter, combine the filtrate, concentrate it into a thick paste with a relative density of 1.15-1.25 (65-70°C), mix it with the above powder Homogenize, make granule, dry, pulverize, sieve, make 1000 capsules, obtain final product; The quality control method of this medicine comprises the following steps:
[0044] (1) Simultaneous determination of amygdalin (C 20 h 27 NO 11 ), paeoniflorin (C 23 h 28 o 11 ), naringin (C 27 h 32 o 14 ) content:
[0045] a. Chromatographic conditions: use octadecylsilane bonded silica gel as filler; acetonitrile-0.8% glacial acetic acid solution is mobile phase gradient elution (gradien...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com