Pharmaceutical composition containing pentoxifylline and carbazochrome sodium sulfonate and application thereof
A technology of pentoxifylline and sodium carboxylate, which is applied in the field of medicine to achieve the effects of reducing rectal vascular permeability, small mutual influence and inhibiting swelling of the right ear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
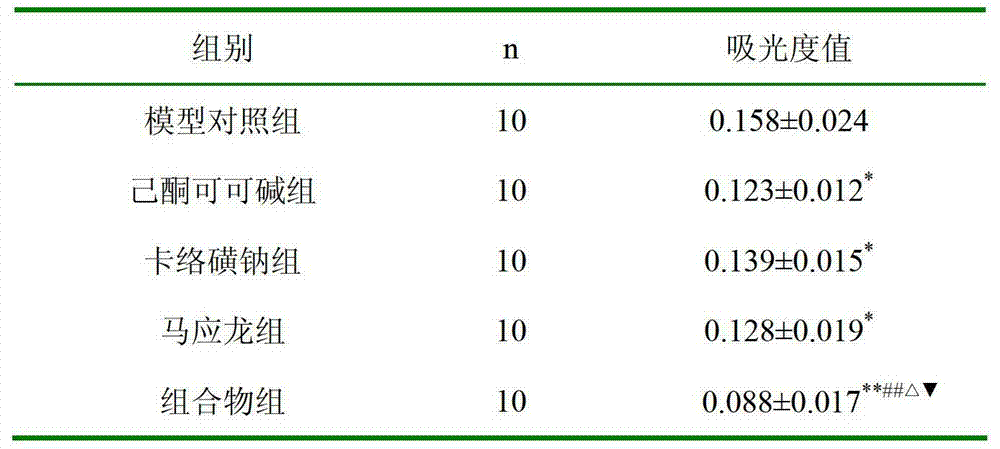
[0023] The experiment of embodiment 1 rat croton oil hemorrhoid model
[0024] experimental method:
[0025] 50 SD rats (about 200 grams) were randomly divided into five groups according to body weight: model control group, pentoxifylline group (2% pentoxifylline glycerin solution, 0.6ml each administration), carbosulfonium sodium group ( 1% carbosulfonate sodium glycerin solution, 0.6ml per administration), composition group (composition glycerol solution, containing 2% pentoxifylline, 1% carbosulfonate sodium, 0.6ml per administration), Mayinglong group (manufactured by Mayinglong Musk Hemorrhoid Ointment Wuhan Mayinglong Pharmaceutical Group Co., Ltd., Z42021920, batch number 080615, specification: 10g / tube), 10 in each group. Intrarectal administration to rats two days in advance, twice a day at 9 o'clock in the morning and 21 o'clock in the evening, soaked with 10 mg cotton balls, and stuffed the cotton balls into the rectum. Cotton balls are present (remove if present)...
Embodiment 2
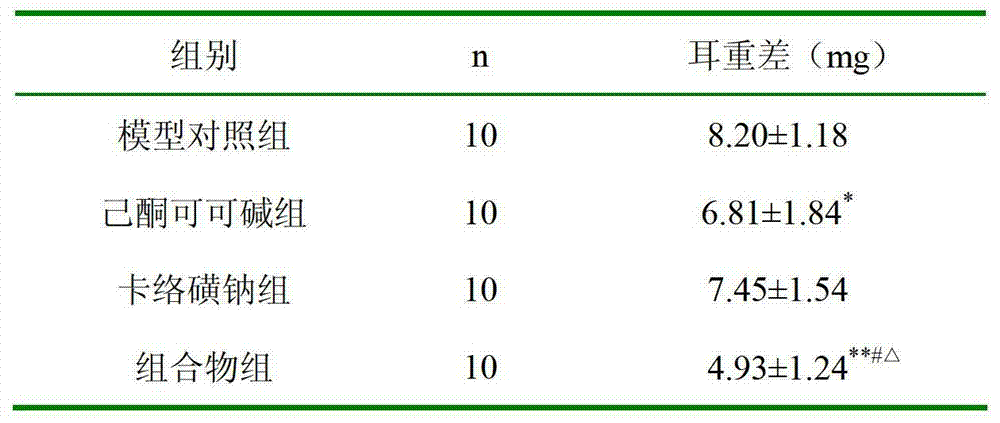
[0036] Example 2 Xylene Anti-inflammatory Experiment in Mice
[0037] Perianal pain caused by perianal inflammation is one of the symptoms of hemorrhoids, and detecting the anti-inflammatory effect of the composition can reflect the therapeutic effect of the composition. In the present invention, the anti-inflammatory effect of the medicine is detected by a mouse xylene anti-inflammatory experiment.
[0038] experimental method:
[0039] 40 KM mice, half male and half female, weighing 25-30 grams, were randomly divided into four groups according to body weight, namely, model control group, pentoxifylline group (1.2% pentoxifylline glycerin solution), carbosulfonium sodium group ( 2% sodium carbosulfate glycerin solution), composition group (glycerin solution of the composition, containing 1.2% pentoxifylline, 2% sodium carbosulfate), 10 rats in each group. Take 20 μl of xylene and apply it to the right ear of each mouse. After 30 minutes, take 0.03ml of each test drug and a...
Embodiment 3
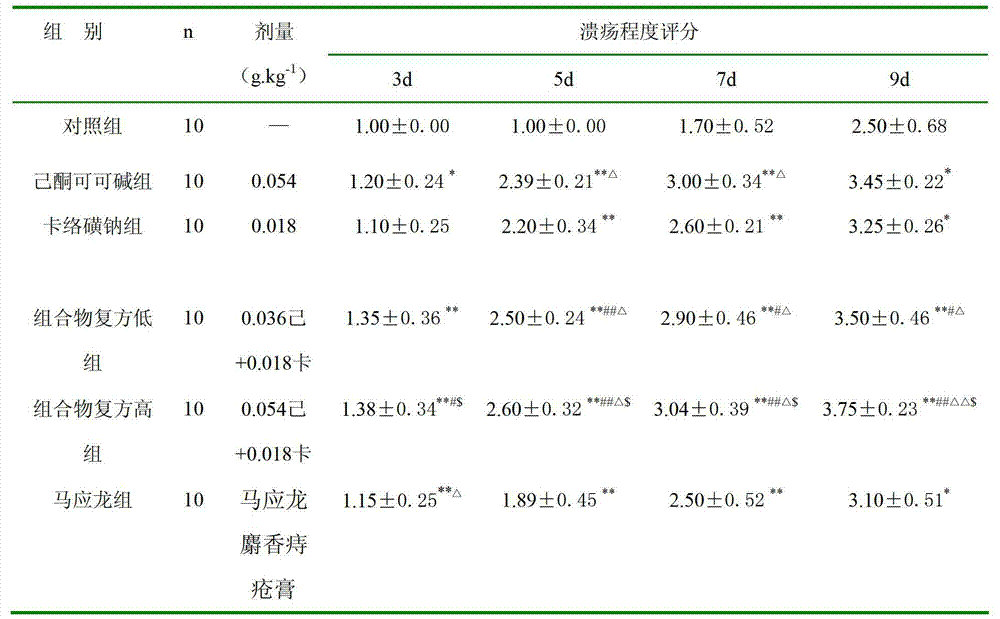
[0046] The effect of embodiment 3 composition on rat perianal ulcer caused by acetic acid
[0047] Take 60 rats, half male and half male, weighing 200-250 g. Take the filter paper and use a puncher with an inner diameter of 6mm to make filter paper pieces of equal size, put them into 99.0% acetic acid solution and soak them fully, put the soaked filter paper pieces around the anus, so that the filter paper pieces are in close contact with the perianal skin and mucous membranes, Use a piece of filter paper each time, change the filter paper piece after 1 min for each rat, and remove the filter paper piece after 0.5 min. On the 2nd day, the rats were randomly divided into 6 groups, 10 in each group, and the high-dose and low-dose groups were given high-concentration (composition glycerin solution, containing 1.8% pentoxifylline + 0.6% carbosulfonium sodium), and low-concentration (composition glycerin solution, 1.2% pentoxifylline + 0.4% sodium carbosulfate solution), the pento...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com