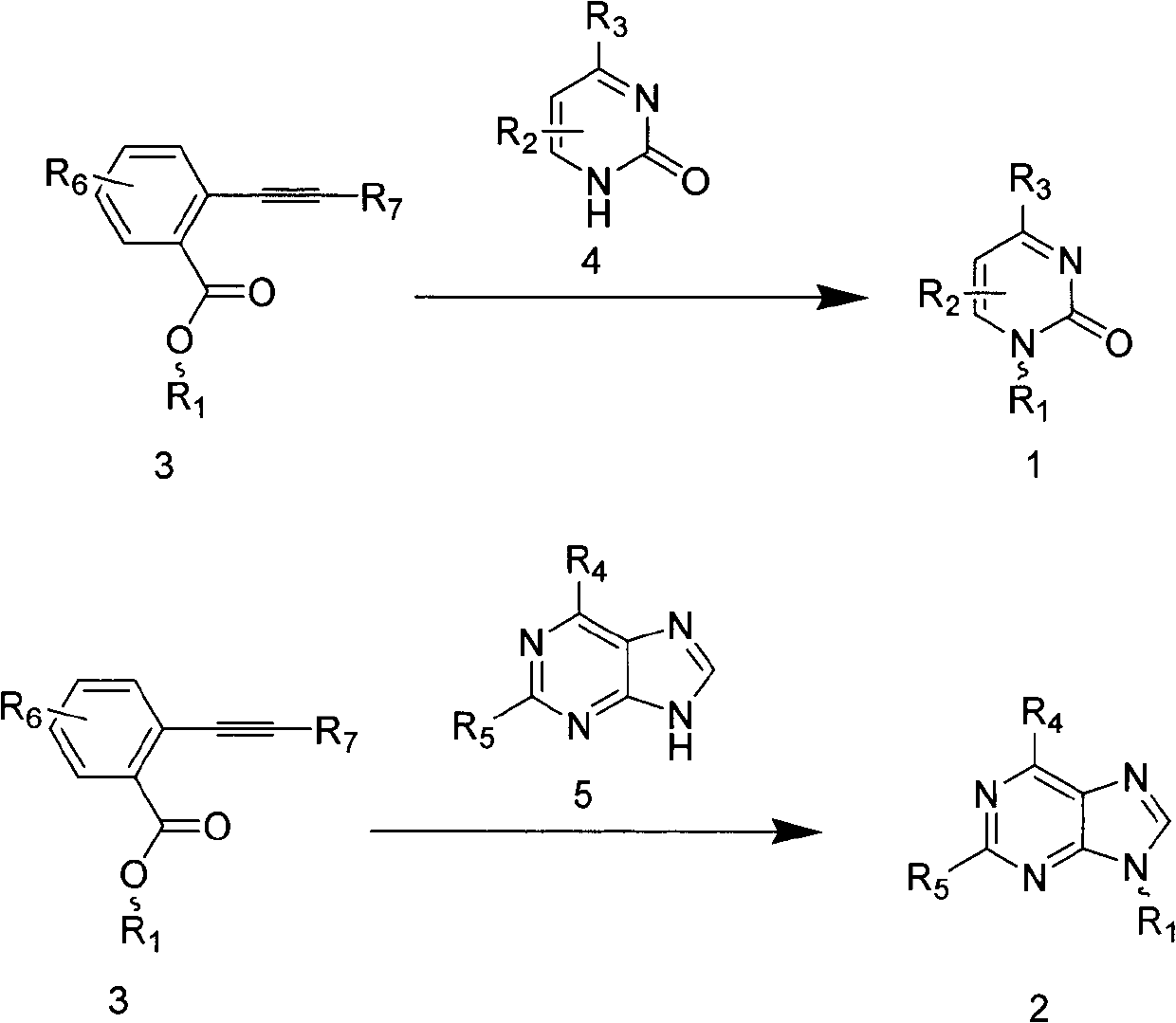
Preparation method of pyrimidine nucleoside compound or purine nucleoside compound
A technology of nucleoside compounds and purines, which is applied in the field of preparation of pyrimidine nucleoside compounds or purine nucleoside compounds, and can solve the problems of severe and harsh reaction conditions and limited types of applicable nucleosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis of whole Bz uridine:
[0052]
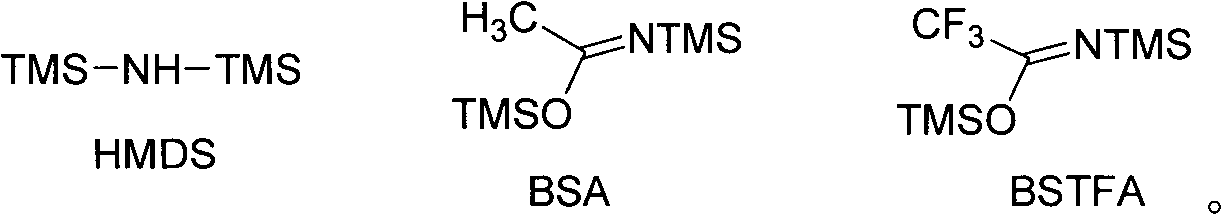
[0053] Reagents and conditions: a) DCC / DMAP, CH 2 Cl 2 , rt; b) BSTFA, AuPPh 3 NTf, CH 3 CN, rt.
[0054] Specific experimental process and data:
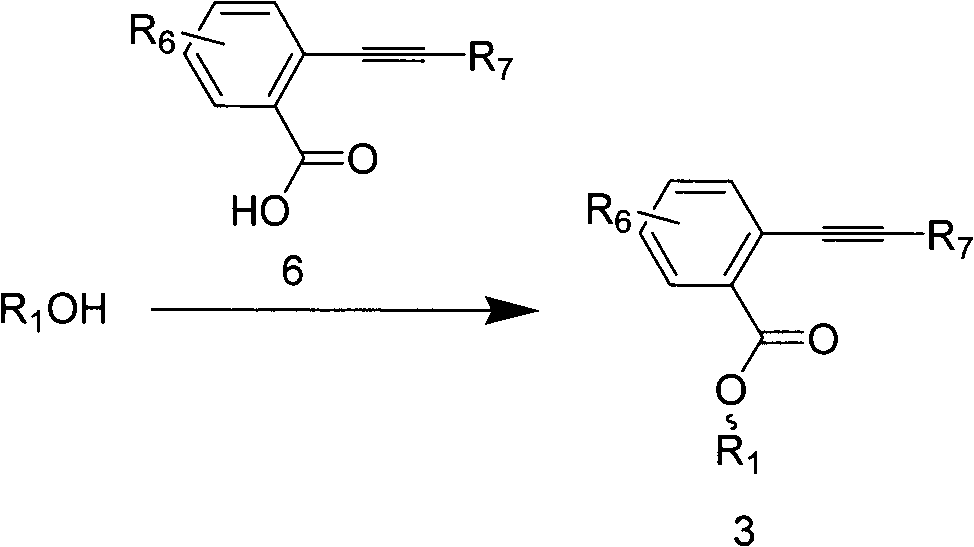
[0055] Under the protection of argon, the anomeric exposed fully Bz-protected ribose (1 g, 2.3 mmol) and o-alkynylbenzoic acid (697 mg, 3.5 mmol) were dissolved in dry CH 2 Cl 2 (2 mL), then DCC (950 mg, 4.6 mmol) and a catalytic amount of DMAP were added to the system and stirred at room temperature for 4 h. The solvent was evaporated to dryness under reduced pressure to obtain a crude product, followed by column chromatography to obtain ribose alkynyl ester donor (1.3 g, 90%).
[0056] The product identification data are as follows: [α] D 25 =+37.6(c1.05, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 ): δ8.05(d, J=7.5Hz, 2H), 7.96(d, J=7.2Hz, 2H), 7.90(m, 3H), 7.63(t, J=7.5Hz, 1H), 7.55(m , 2H), 7.48(m, 4H), 7.35(m, 5H), 6.68(s, 1H), 6.04(m, 2H), 4.88(m, 1H), 4.76(dd, J=12.6, 5...
Embodiment 2
[0060] Synthesis of glucothymidine:
[0061]
[0062] Reagents and conditions: a) EDC / DIEPA, CH 2 Cl 2 , rt; b) BSTFA, AuPPh 3 NTf, CH 3 CN, rt.
[0063] Specific experimental process and data:
[0064] The anomeric exposed whole Bz glucose (2160mg, 4.0mmol), alkynoic acid (970mg, 4.8mmol, 1.2eq), DMAP (488mg, 4.0mmol, 1.2eq), EDCI (955mg, 5.0mmol, 1.25eq) and DIPEA (1.3mL, 7.2mmol, 1.8eq) was put into a 25mL egg bottle, injected with 4mL dry DCM, stirred at room temperature for 3h, diluted with DCM, washed with saturated saline, dried over anhydrous sodium sulfate, filtered, concentrated, and flash column chromatography (PE / EA=5:1), the product (2815 mg, 97%, β / α=1:1.3) was obtained.
[0065] The product identification data are as follows: α configuration: [α] D 27 =98.3(c 4.1, CHCl 3 ). 1 HNMR (300MHz, CDCl 3 ): δ8.097.22(m, 24H), 6.93(d, 1H, J=3.3Hz), 6.32(t, 1H, J=10.2Hz), 5.90(t, 1H, J=9.9Hz), 5.73(dd , 1H, J=10.2, 2.7Hz), (m, 2H), 4.52(dd, 1H, J=12.3, 3...
Embodiment 3
[0070] Synthesis of glucocytosine:
[0071]
[0072] Reagents and conditions: a) EDC / DIEPA, CH 2 Cl 2 , rt; b) BSTFA, AuPPh 3 NTf, CH 3 CN, rt.
[0073] Specific experimental process and data:
[0074] The preparation method of the whole acetyl group-protected glucoynyl ester donor is the same as that of the whole benzoyl-protected glucose.
[0075] The product identification data are as follows: α configuration: [α] D 29 =96.7(c 2.5, CHCl 3 ). 1 H NMR (300MHz, CDCl 3 ): δ7.96(d, 1H, J=8.1Hz), 7.59(d, 1H, J=7.8Hz), 7.51(t, 1H, J=7.5Hz), 7.41(t, 1H, J=7.5 Hz), 6.63(d, 1H, J=3.6Hz), 5.62(t, 1H, J=9.6Hz), 5.25(m, 2H), 4.33(d, 1H, J=9.6Hz), 4.12(d, 1H, J=10.2Hz), 4.26(m, 1H), 2.10(s, 3H), 2.05(s, 3H), 2.04(s, 3H), 2.01(s, 3H), (m, 4H), 0.96(t, 1H, J=7.2Hz). 13 C NMR (75MHz, CDCl 3 ): δ170.5, 170.0, 169.6, 169.2, 163.9, 135.0, 132.3, 130.6, 129.6, 127.2, 125.1, 97.0, 89.7, 79.5, 70.1, 69.9, 69.2, 67.8, 61.2, 30.6, 21.9, 20.5, 2 20.3, 19.4, 19.0, 13.5. HRMS (MALDI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com