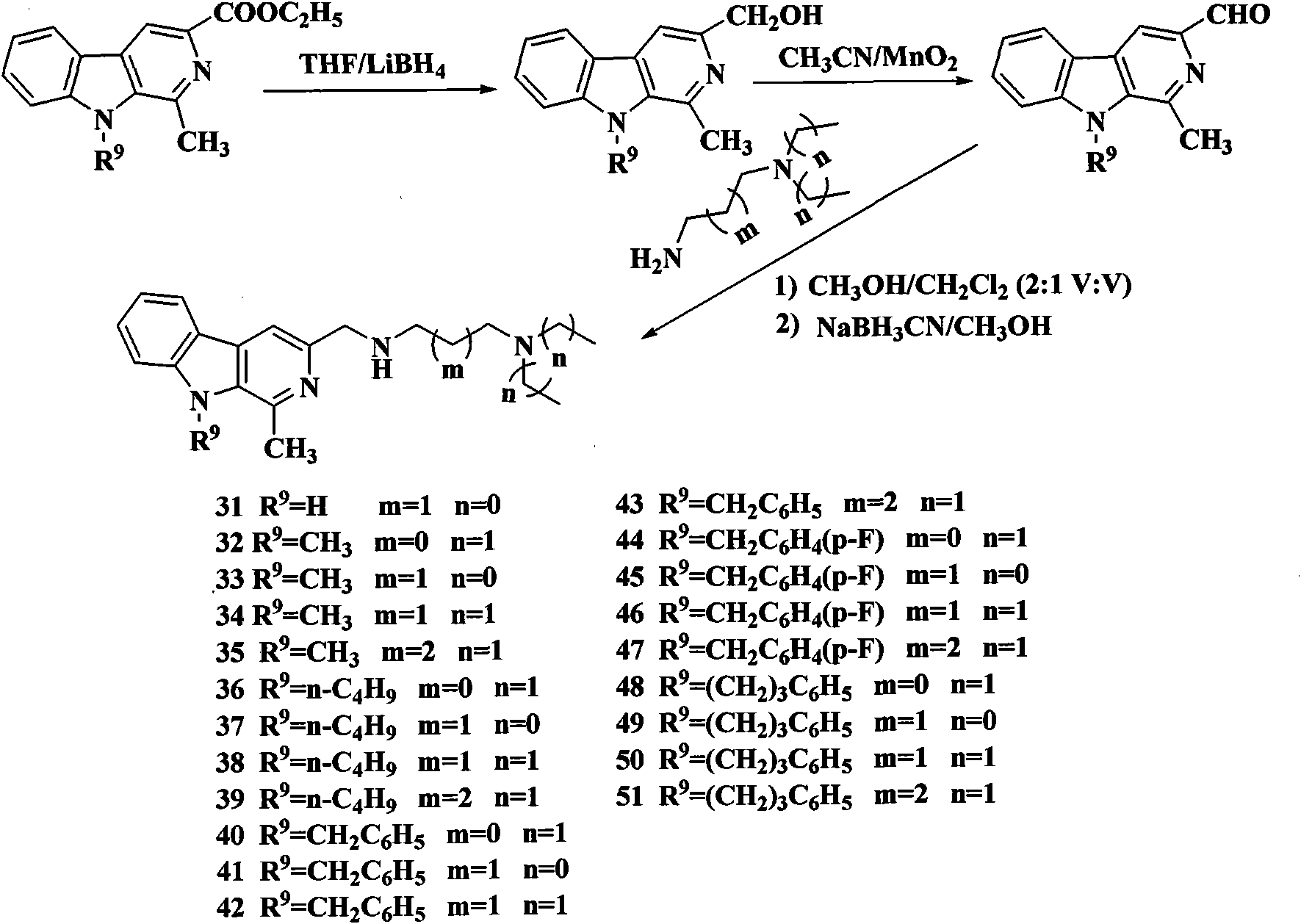Beta-carboline alkali derivative compounds and application thereof
A technology of carboline bases and derivatives, which is applied in the field of compounds of general formula β-carboline base derivatives, which can solve the problem of insufficient anti-tumor activity, limited application prospects, poor water solubility of β-carboline base derivatives, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
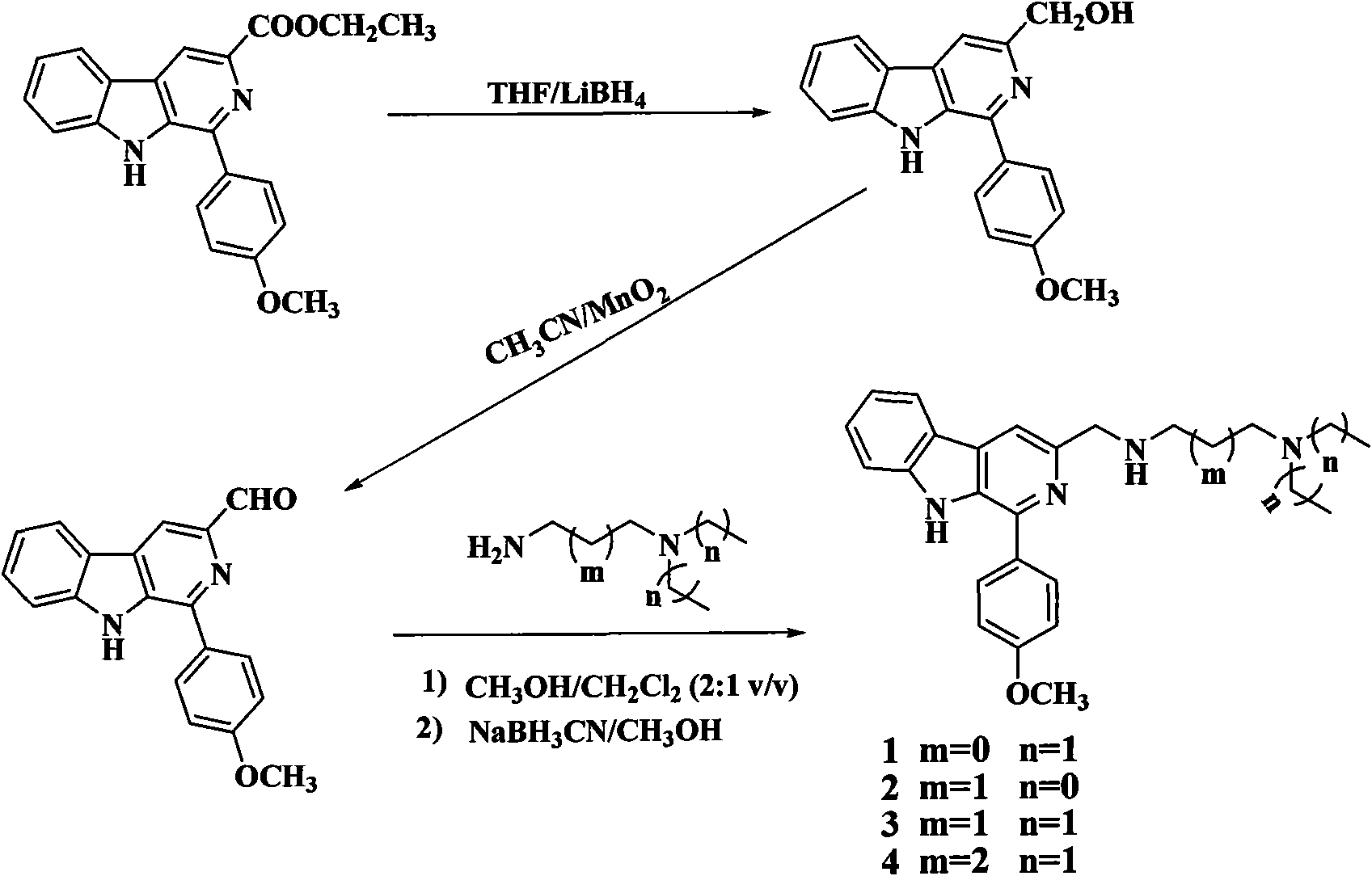
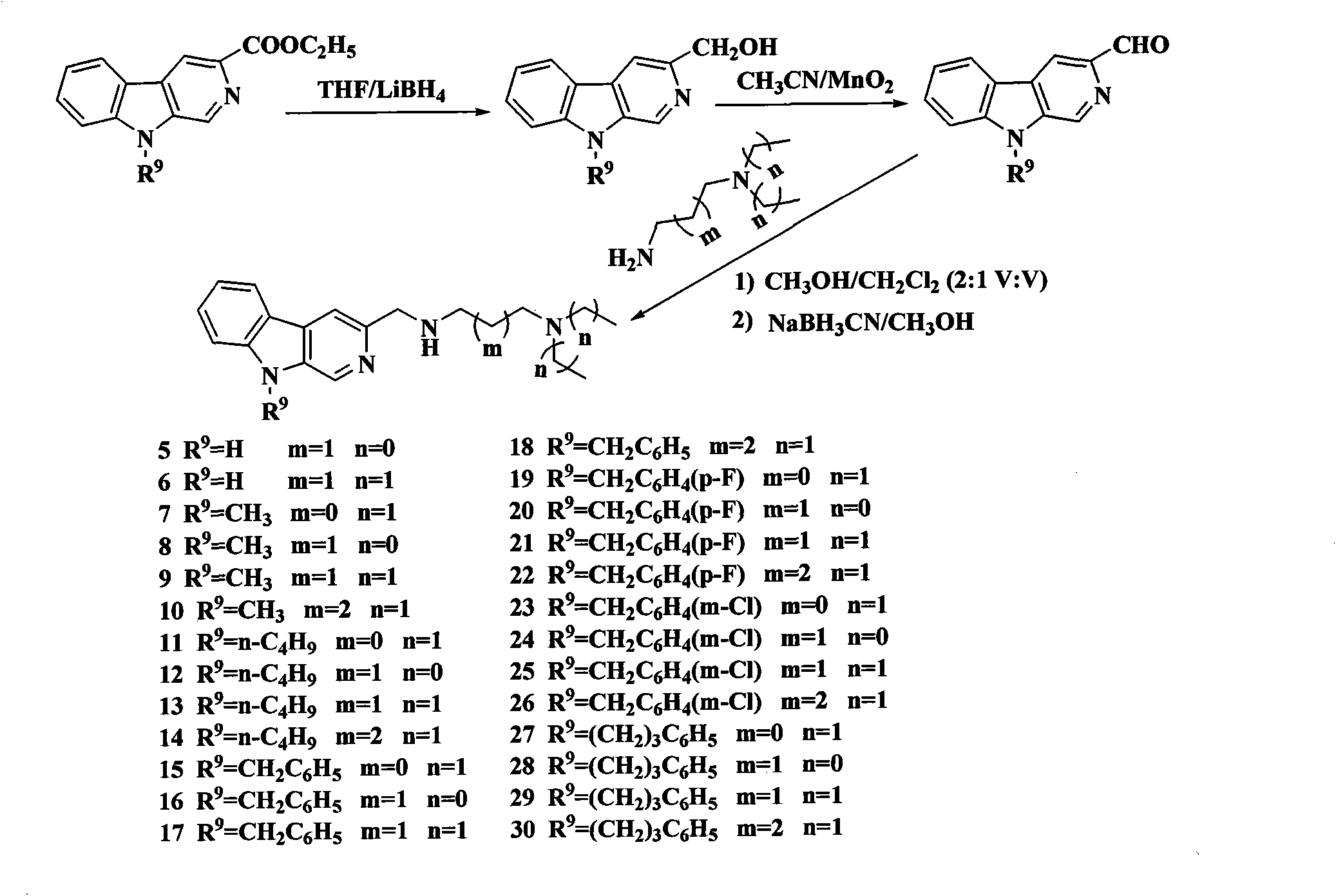
[0121] Example 11 Preparation of-(4-methoxy)phenyl-3-[N-(2-diethylamino)-ethyl]-methylamino-β-carboline (Compound 1)
[0122] The starting material, ethyl 1-(4-methoxy)phenyl-β-carboline-3-carboxylate, was synthesized according to literature methods.
[0123] Step (a): Preparation of 1-(4-methoxy)phenyl-3-hydroxymethyl-β-carboline:
[0124] Ethyl 1-(4-methoxy)phenyl-β-carboline-3-carboxylate (10mmol), THF (200ml), LiBH 4 (30 mmol) was mixed, the reaction was stirred at room temperature for 12 h, followed by TLC (petroleum ether / acetone=1 / 1) for tracking detection, the reaction was completed, the reaction mixture was slowly poured into 200 ml of ice water, stirred for 10 minutes, and concentrated hydrochloric acid was added dropwise to pH. 2-3, stirred at room temperature for 2 h, the mixture was cooled with ice water, the pH value was adjusted to 9-10 with sodium hydroxide solution, extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhyd...
Embodiment 2
[0131] Example 21 Preparation of-(4-methoxy)phenyl-3-[N-(3-dimethylamino)-propyl]-methylamino-β-carboline (compound 2)
[0132] The preparation method is the same as that in Example 1, but in step (c), N,N-dimethylpropanediamine is used as a raw material. A yellow oil was obtained, which was the target compound 2, and the yield was 42%; IR (KBr, cm -1 )v: 2947, 2744, 1621, 1510, 1464, 1378, 1259, 1181, 1024, 756; 1 H NMR (500MHz, D 2 O): δ8.20(s, 1H), 7.93(d, J=8Hz, 1H), 7.63(d, J=9Hz, 2H), 7.44-7.48(m, 1H), 7.35(d, J=8.5 Hz, 1H), 7.15-7.18(m, 1H), 7.03(d, J=8.5Hz, 2H), 4.64(s, 2H), 3.77(s, 3H), 3.32-3.35(m, 2H), 3.26 -3.30(m, 2H), 2.90(s, 6H), 2.22-2.28(m, 2H); 13 C NMR (100MHz, D 2 O+Dioxane): δ161.9, 143.4, 139.0, 132.7, 131.8, 131.5, 131.0, 130.8, 122.5, 122.0, 120.9, 119.3, 117.1, 115.1, 112.4, 55.6, 54.2, 47.7, 44.8, 42.9, 21.3; ESI -MS m / z: 389.3(M+1) + .
[0133] The preparation method of compound 2 hydrochloride is the same as that in Example 1.
Embodiment 3
[0134] Example 3. Preparation of 1-(4-methoxy)phenyl-3-[N-(3-diethylamino)-propyl]-methylamino-β-carboline (compound 3)
[0135] The preparation method is the same as that in Example 1, but in step (c), N,N-diethylpropanediamine is used as the raw material. A yellow oil was obtained, which was the target compound 3, and the yield was 50%; IR (KBr, cm -1 )v: 2941, 2740, 1628, 1510, 1449, 1380, 1257, 1181, 1025, 756; 1 H NMR (500MHz, D 2 O): δ8.34(s, 1H), 8.11(d, J=8.5Hz, 1H), 7.78(d, J=9Hz, 2H), 7.62(t, J=7Hz, 1H), 7.51(d, J=8Hz, 1H), 7.33(t, J=7.5Hz, 1H), 7.19(d, J=8.5Hz, 2H), 4.75(s, 2H), 3.92(s, 3H), 3.43(t, J =7.5Hz, 2H), 3.29-3.36 (m, 6H), 2.28-2.34 (m, 2H), 1.35 (t, J=7.5Hz, 6H);13 C NMR (125MHz, D 2 O): δ162.3, 143.8, 139.6, 133.2, 132.2, 132.1, 131.6, 131.2, 122.9, 122.4, 121.6, 119.8, 117.4, 115.5, 112.9, 56.1, 48.8, 48.2, 48.0, 45.3, 21.2, 8.6; ESI -MS m / z: 417.4(M+1) + .
[0136] The preparation method of compound 3 hydrochloride is the same as that of Example...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com